Group b Neisseria meningitidis recombinant pili protein fim and its preparation method and application
A technology for Neisseria meningitidis and meningitis, which is applied in the field of Neisseria meningitidis recombinant fimbriae protein Fim of group B and its preparation, can solve the problems of vaccine research and marketing, and achieves good repeatability, high recovery rate, and resistance to The effect of breaking the adhesion of Neisseria meningitidis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Further, the present invention also provides a method for preparing the above-mentioned Neisseria meningitidis recombinant pilus protein Fim, comprising the following steps:
[0054] a. Plasmid construction
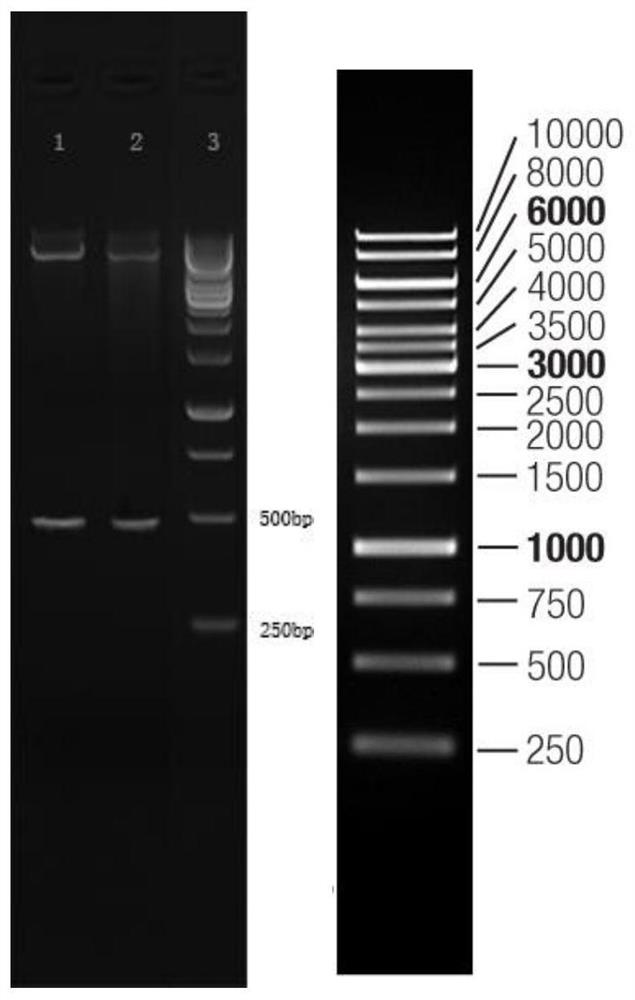
[0055] The gene whose nucleotide sequence is SEQ ID NO:1 is connected in the expression vector plasmid, the plasmid is constructed and transferred into the host bacteria for induced expression;
[0056] b. Sonication, centrifugation
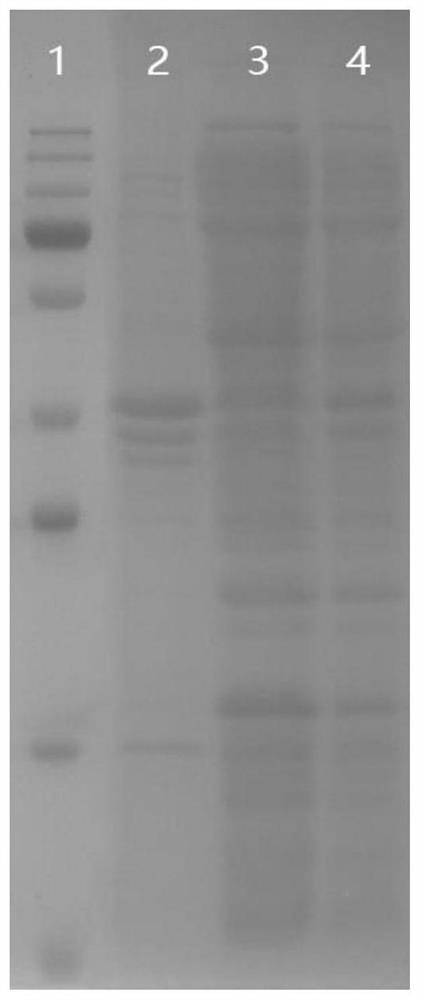
[0057] The bacteria obtained in the collection step a were resuspended and mixed evenly with the bacteriostasis solution, and after an ice-water bath, the bacteria were destructed by ultrasonication, centrifuged at a high speed, and the supernatant was collected;
[0058] c. Ni column affinity purification
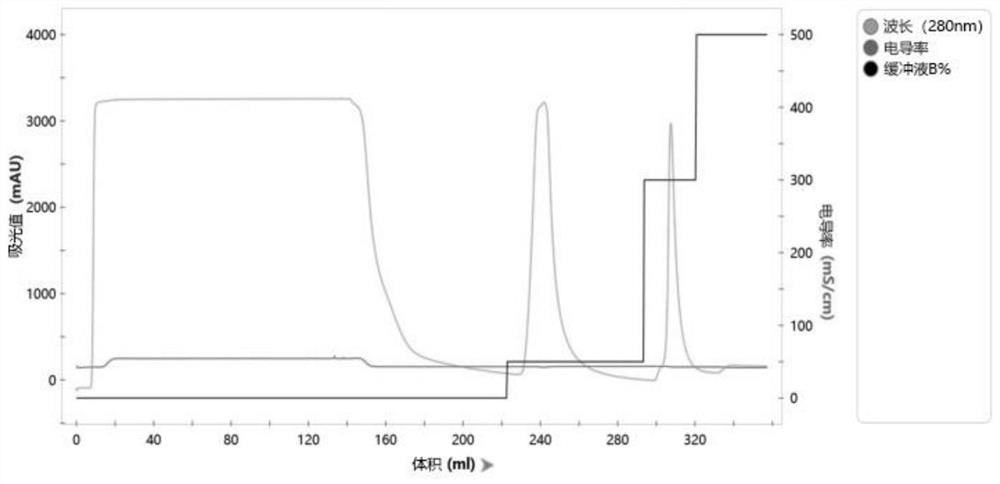
[0059] Preliminary purification with Ni affinity packing, use liquid A to equilibrate the chromatographic column, and use liquid B to elute;
[0060] d. SP column affinity chromatography;
[0061] The target protein purified i...
Embodiment 1
[0080] Construction and identification of the recombinant plasmid pET28a / Fim of embodiment 1 Fim gene
[0081] The specific operation steps are as follows:
[0082] (1) According to the complete genome sequence of Neisseria meningitidis group B, the candidate antigen Fim (SEQ ID NO: 2) with protective immune response was screened by reverse vaccinology.
[0083] (2) According to the amino acid sequence of Fim, Escherichia coli partial tropism codon optimization was performed to obtain the target gene fragment, the sequence is SEQ ID NO: 3 (the base sequence of the restriction site is underlined).
[0084] Nucleotide sequence of SEQ ID NO:3 target gene
[0085] ccatggcggaaggccagaaaagcgcggtgaccgaatattatctgaaccacggagaatggccgggcaacaacagcagcgcgggcgtggcgaccagcgcggatattaaaggcaaatatgtgaaaagcgtggaagtgaaaaacggcgtggtgaccgcgcagatggcgagcagcaacgtgaacaacgaaattaaaggcaaaaaactgagcctgtgggcgaaacgtcaggcgggcagcgtgaaatggttttgcggcctgccggtgacccgtgcggataacgcgaaagatgatgcggtgaccgcggcggcgaccggcaccgataa...
Embodiment 2
[0097] Example 2 Induced expression, purification and identification of expression form of recombinant pilus protein Fim in prokaryotic expression system-Escherichia coli The specific operation steps are as follows:
[0098] (1) Take 100 μl of overnight cultured pET28a / Fim / BL21(DE3) bacterial solution and add it to 10 mL of kanamycin + resistant LB medium, culture overnight at 220 rpm at 37°C, take 200 μl of overnight cultured bacterial solution and add 20 mL card In the LB medium with Namycin + resistance, culture at 220rpm 37℃ for 2h, after secondary activation to OD600 of 0.8, add IPTG Isopropyl-β-D-thiosemi lactoside 10 μl to make the final concentration 0.5 mM, and then placed on a shaker at 220 rpm at 37° C. to induce expression for 4 hours.
[0099] (2) Take out the bacterial solution after induced expression, centrifuge at 5000g for 15min, discard the supernatant, add 3ml of bacteriostasis solution (50mM PB, 0.3M NaCl, pH7.4) and mix evenly, ultrasonically lyse in i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com