Compound and application thereof
A technology of compounds and uses, applied in the field of fluorescence imaging, can solve problems such as limited clinical translation and unclear long-term toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
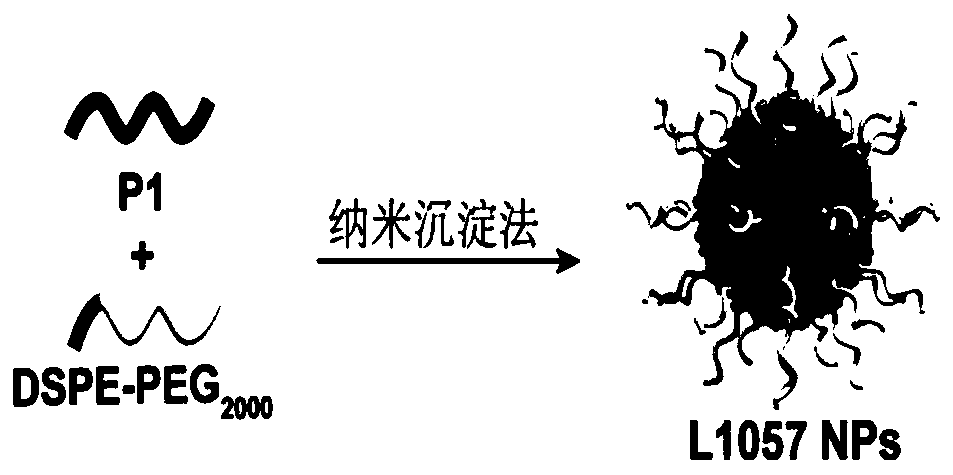
[0087] The conjugated polymer is firstly prepared, and the corresponding composition is prepared later by a nano-co-precipitation method.
[0088] Add monomer 1 (134.5mg, 0.1mmol), monomer 2 (69.8mg, 0.1mmol), catalyst Pd in a 50mL Schlenk tube 2 (dba) 3 (3mg, 3.3μmol) and P(o-tolyl) 3 (3 mg, 9.8 μmol) and solvent toluene (10 mL). The reaction mixture was reacted at 100° C. for 2 h under the protection of argon atmosphere. The reaction was then stopped, cooled, and the product was precipitated in methanol. The crude product was then dissolved in dichloromethane (200 mL), and extracted with water (100 mL×3), Na 2 SO 4 Dry and filter. Finally, the organic phase was concentrated to ~10 mL, and added dropwise to methanol (100 mL), and P1 was obtained by precipitation and filtration as a brown solid (104 mg, yield: 67%).
[0089] 1 HNMR (CDCl 3 , 400MHz, ppm) δ: 9.52-7.50(m), 7.78-7.76(m), 7.63(br), 7.57-7.55(d), 7.33-7.28(m), 7.22-7.17(m), 7.11-7.03 (m), 6.65(br), 3.95...
example 2
[0093] The conjugated polymer is prepared first, and then the corresponding compound is prepared through chemical modification.
[0094] The synthesis method of polymer 4 is similar to that of P1 in Example 1, but the side chain of polymer 4 contains an azide (N3) functional group, which can perform a click chemical reaction with a substrate containing an alkyne bond.
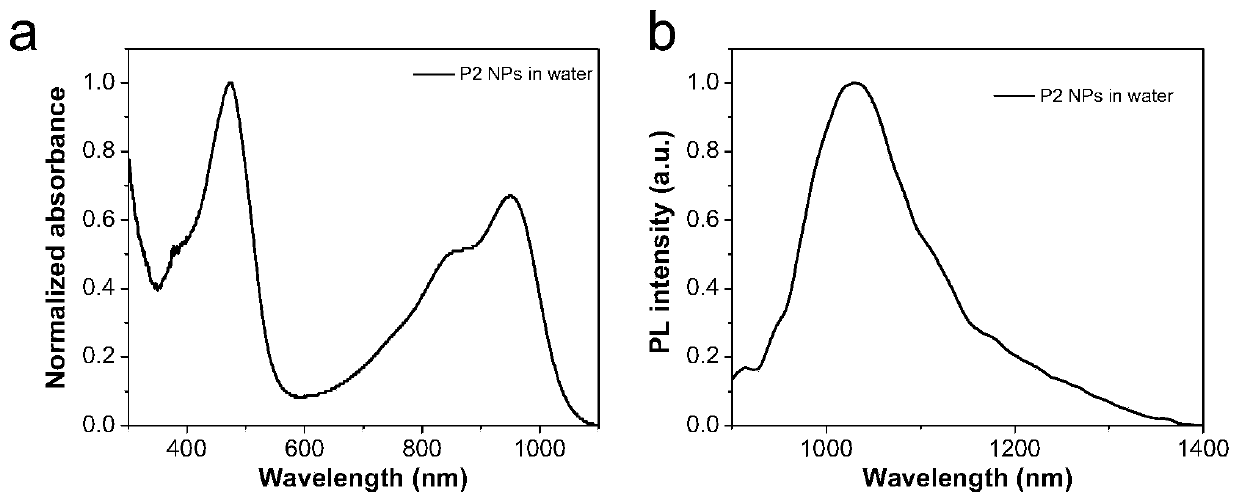
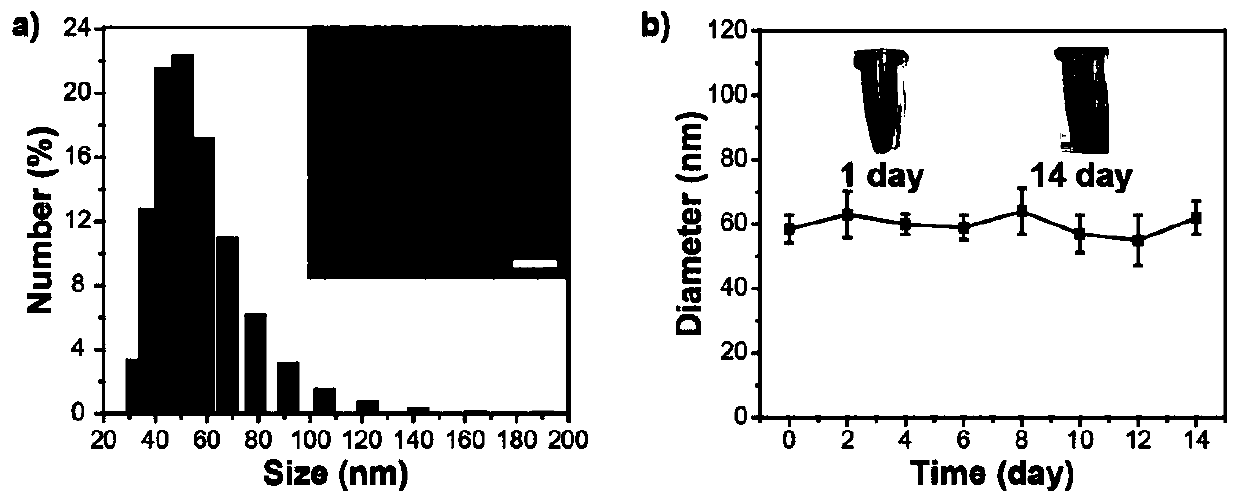
[0095]Synthesis example of polymer P2: add reactants in a 50mL reaction tube, polymer 4 (50mg), polyethylene glycol 2000 with acetylene bond (146mg), catalyst copper sulfate (5mg), sodium ascorbate (5mg), solvent tetrahydrofuran (50mL). React at room temperature for 48 hours under an argon atmosphere, and then dialyze in water with a dialysis membrane with a molecular weight cut-off of 12,000-14,000 to remove unreacted polyethylene glycol 2000 with acetylenic bonds and catalyst, and then freeze-dry to obtain flocculent solid Polymer P2.
[0096] 1 HNMR (CDCl 3 ,400MHz,ppm):9.52(br),7.78(br),7.65-7.57(br),7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Laser power | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com