Fluorescent protein, and preparation method and application thereof
A fluorescent protein and fusion protein technology, applied in the field of fluorescent protein and its preparation, can solve problems such as poor fusion performance, poor photobleaching, and unfavorable construction of molecular probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Preparation and detection of fluorescent protein mCyRFP2
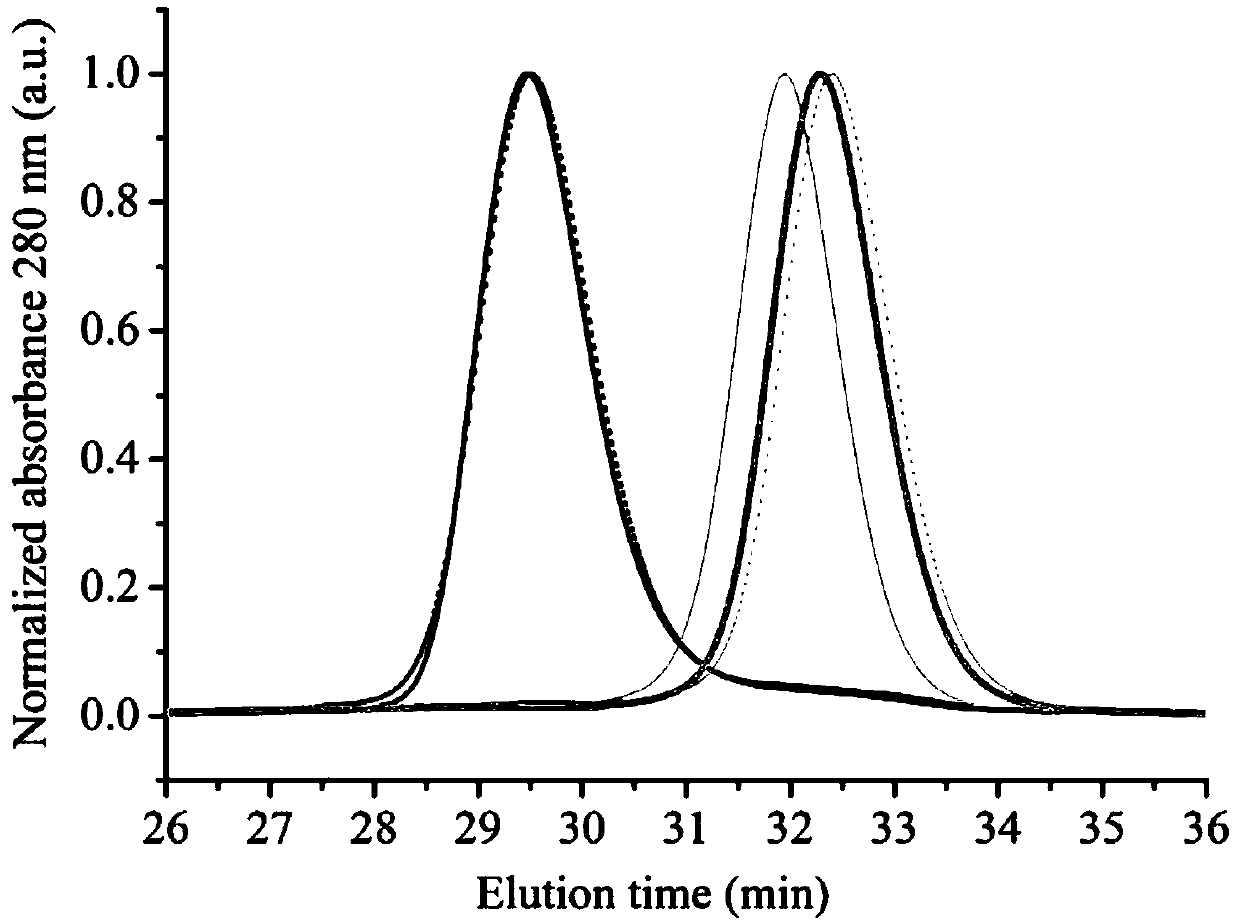
[0087] The red fluorescent protein CyOFP1 was subjected to site-directed mutagenesis, and then the mutants were expressed and screened on the constitutive expression vector pNCS, and the expression strain used was XL-10Gold (purchased from Agilent Technologies). In order to ensure the integrity of the library, 10 clones were set up for each mutant, and finally the fluorescent properties of the mutant were detected by visual resolution and blue LED excitation light through an orange acrylic filter, and were detected by high performance liquid chromatography HPLC (LC-20A ( SHIMADZU)) detect protein monomer, the molecular sieve column used is Superdex 200 10 / 300GL column (GE Bioscience), screen out the single clone expressing monomeric red fluorescent protein (such as figure 1 shown), the fluorescent protein was named mCyRFP2. The sequencing results show that the fluorescent protein mCyRFP2 provided in t...
Embodiment 2
[0104] Example 2 Monomer identification of fluorescent protein mCyRFP2
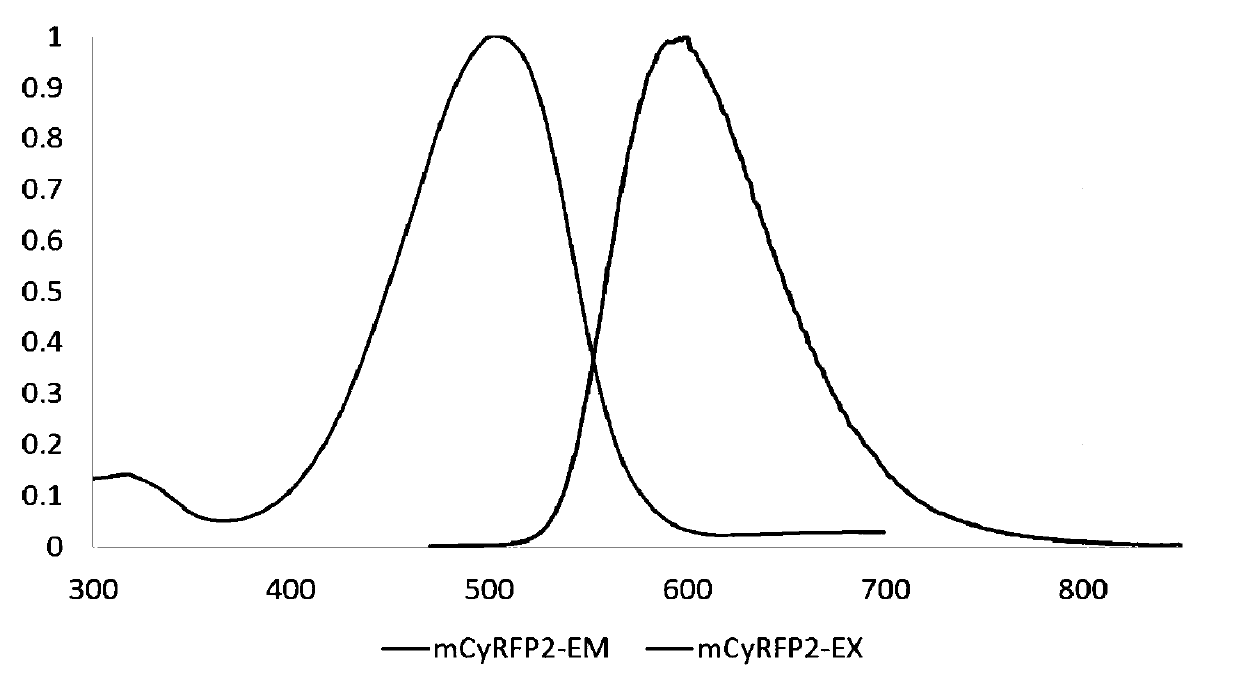
[0105] (1) Bacteria expressing mCyRFP2 were lysed using B-PER II (purchased from Pierce), and then HisPur Cobalt Resin (purchased from Pierce) was used to purify the protein, followed by an Econo-Pac 10DG gravity flow chromatography column (purchased from U.S. Bio-Rad company) to desalt. After completing the above protein purification steps, use Lambda35UV / VISand LS-55 fluorescence spectrometer (purchased from Perkin Elmer) to detect the absorption spectrum, excitation spectrum and emission spectrum of mCyRFP2.
[0106] The result is as figure 2 As shown, the protein sample concentration is 10mg / mL, and the flow rate is 0.5mL / min (FusionRed is the best monomeric red fluorescent protein known so far, and mKate2 is a dimer fluorescent protein known so far). The black dotted line represents mKate2, the black solid line represents CyOFP1, the gray solid line represents FusionRed, the gray dotted line repre...
Embodiment 3
[0110] Example 3 Identification of the photostability of the fluorescent protein mCyRFP2
[0111] The genes containing mCyRFP2, CyOFP1, mCyRFP1 and mNeonGreen were respectively integrated into the nucleus-localized plasmids and transfected into HeLa cells by Lipofectamine 2000 kit (purchased from GE Healthcare Dharmacon). A two-photon upright fluorescence microscope (A1MP, Nikon Instruments, numerical aperture 20×0.5) was used to excite the nuclei containing mCyRFP2, CyOFP1, mCyRFP1 and mNeonGreen proteins with 920 nm excitation light, and the excitation energy used was 10 mM. Draw a curve of fluorescence decay with the extension of excitation time, the result is as follows Figure 4 As shown in the figure, it can be seen that with the prolongation of the illumination time, the fluorescence intensity values of the two proteins CyOFP and mCyRFP1 decreased rapidly, but the fluorescence intensity values of the two proteins mCyRFP2 and mEGFP decreased slowly. Even after 110 sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com