Method for synthesizing R-3-(2-chloro-1-hydroxyethyl) phenol, phenylephrine and eye drops
A hydroxyethyl, R-3- technology, applied in chemical instruments and methods, botanical equipment and methods, preparation of organic compounds, etc., can solve problems such as complex steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] An Escherichia coli expressing ketoreductase, said ketoreductase is a reductase encoded by a ketoreductase gene, the amino acid sequence of said ketoreductase is shown in SEQ ID No.2, said ketoreductase gene The nucleotide sequence is shown in SEQ ID No.1.
[0072] Specifically, the construction and culture method of the Escherichia coli expressing ketoreductase are as follows:
[0073] 1) Digest the artificially synthesized DNA fragment of the ketoreductase gene with restriction endonuclease NdeI and restriction endonuclease EcoRI at 37°C for 8 h, purify by agarose gel electrophoresis, and use agarose gel DNA The recovery kit recovers the target fragment;
[0074] 2) Under the action of T4 DNA ligase, the target fragment is ligated with the plasmid pET24a that has also been digested with restriction endonuclease NdeI and restriction endonuclease EcoRI at 25°C, and the ligated product is transformed overnight Obtain recombinant Escherichia coli;
[0075] 3) Inoculate...
Embodiment 2
[0077] The thalline prepared in Example 1 was lysed back with 0.1 mol / L PBS buffer solution (pH=7.0), homogeneously crushed, centrifuged to collect the supernatant of the enzyme solution and freeze-dried to obtain ketoreductase (enzyme powder) , wherein, the added amount of the PBS buffer is added according to the weight ratio of bacteria: PBS buffer = 1:5.
Embodiment 3
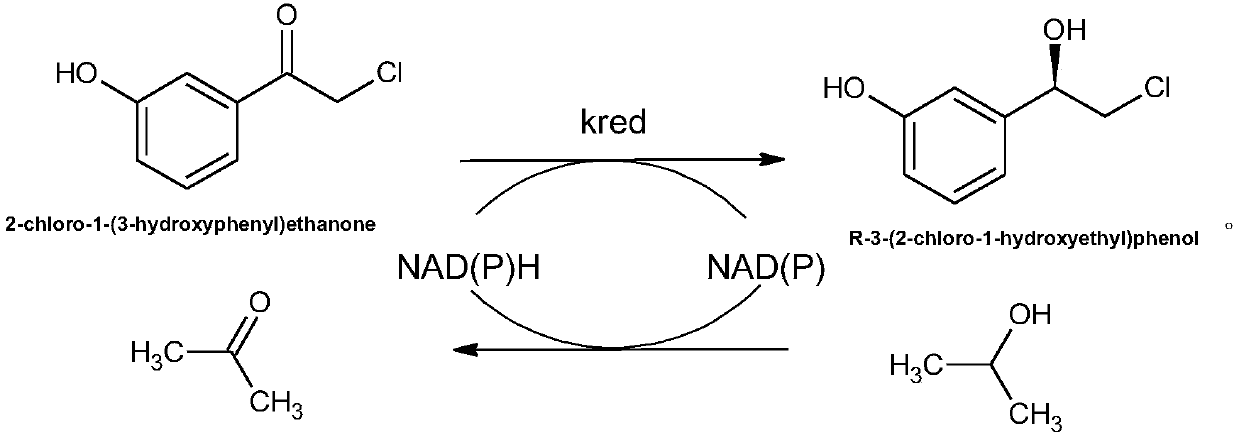
[0079] The ketoreductase prepared in Example 2 is used as a catalyst to catalyze the synthesis of R-3-(2-chloro-1-hydroxyethyl)phenol, specifically, the catalytic synthesis of R-3-(2-chloro-1- The method of hydroxyethyl) phenol comprises the following steps: with 2-chloro-1-(3-hydroxyphenyl) ethanone as substrate, with the ketoreductase prepared in embodiment 2 as catalyst, adding hydrogen donor, buffer Liquid and coenzyme NAD form a reaction system to carry out catalytic reaction in a reaction bottle. Specifically, add magnets to the reaction bottle and place it on a reactor preheated to 30°C, and adjust the reactor speed to 400rpm for stirring reaction. 24 After 1 hour, 5 mL of acetonitrile was added to terminate the reaction to obtain a reaction solution.
[0080] In the embodiment of the present invention, the phenylephrine intermediate is R-3-(2-chloro-1-hydroxyethyl)phenol.
[0081] In the embodiment of the present invention, the total volume of the catalytic system is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com