Thioketal bond connected pegylated Ce6 material as well as preparation method and application thereof
A technology of PEGylation and polyethylene glycol carboxyl group is applied in the field of PEGylated Ce6 material and its preparation, which can solve problems such as the limitation of wide applicability of nanoparticles, achieve good biocompatibility and degradability, The effect of enhanced uptake, great potential for clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
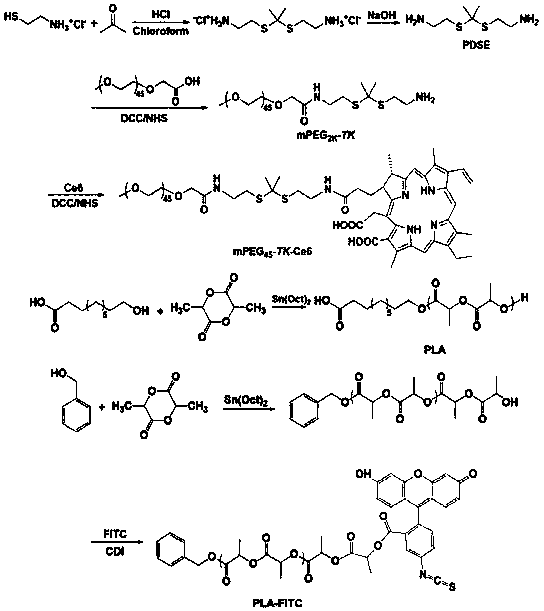
[0074] Embodiment 1, mPEG 45 -Synthesis and Characterization of TK-Ce6 and PLA
[0075] 1. Pegylated Ce6 materials linked by thioketal bonds (mPEG 45 -TK-Ce6) and polylactic acid (PLA) synthesis
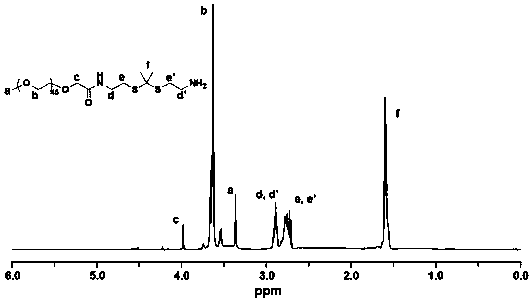
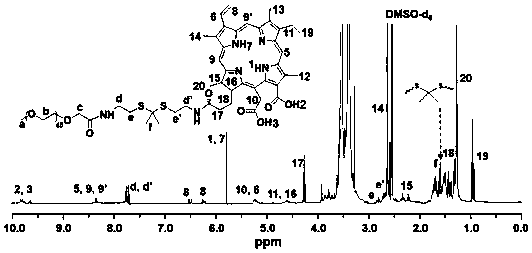
[0076] mPEG 45 -TK-Ce6 consists of 2,2'-(propane-2,2-diylbis(sulfadiyl))bis(ethanol-1-amine) and mPEG 45 -COOH, Ce6 can be obtained through condensation reaction.
[0077] PLA is obtained by using stannous isooctanoate as a catalyst, and 10-hydroxydecanoic acid triggers the ring-opening polymerization of L-lactide.
[0078] Synthetic route such as figure 1 shown.
[0079] Preparation and pretreatment of required components
[0080] 1. Synthesis of PEGylated Ce6 materials linked by thioketal bonds
[0081] (1) Cysteamine hydrochloride (11.36g, 100mmol) and anhydrous acetone (15.6g, 270mmol) were mixed and dissolved in 4 mL of chloroform, and dry hydrogen chloride was introduced to saturate the solution and stirred at room temperature for 8 hours. After the reaction, the white...
Embodiment 2
[0091] Example 2, Preparation and Application of Photoinduced ROS Responsive Nanoparticles
[0092] 1. Preparation of nanoparticles encapsulating Pt(IV) prodrugs (TCNP Pt )
[0093] The above mPEG 45 -TK-Ce6 (5.0 mg), PLA (5.0 mg) and Pt(IV) (0.5 mg) were dissolved in dimethyl sulfoxide (DMSO, 1.0 mL), and then added dropwise to ultrapure water (10.0 mL) Stir for 2 hours. This solution was dialyzed with ultrapure water for 24 hours, and filtered with a 0.45 μm filter membrane to obtain the nanoparticles (abbreviated as TCNP) of the encapsulated Pt (IV) prodrug Pt ) solution. Similarly, fluorescein isothiocyanate (FITC)-labeled Pt(IV) prodrug-encapsulated nanoparticles (abbreviated as FITC TCNP Pt ) solution.
[0094] 2. Generation of ROS in vitro
[0095] The above TCNP Pt solution (2mL), free Ce6 solution ([Ce6]=10μg / mL, 2mL), 50mM p-nitrosodimethylaniline (RNO, 100μL) and 100mM imidazole (100μL) in 20 mM phosphate buffer (pH 7.4 ) and mixed at 0.05W / cm 2 660 nm la...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com