Ophthalmic composition and preparation method thereof
A composition and ophthalmology technology, applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve unfavorable treatment of dry eye, damage to ocular surface cells, tear film instability, etc. problem, achieve the effect of protecting ocular surface cells, reducing tear evaporation, and relieving visual fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The application provides a preparation method of an ophthalmic composition, comprising the following steps:
[0059] Step 1: Dissolve hyaluronic acid salts of different molecular weights in water under heating conditions, add sodium chloride and stir evenly, adjust the pH to alkaline with inorganic alkali or salt, and obtain compound hyaluronic acid through alcohol precipitation, washing and drying salt powder;
[0060] Step 2: Dissolving the compounded hyaluronate powder in water, and then adding zinc hyaluronate, ectoine or ectoine derivatives to obtain a mixed solution;
[0061] Step 3: adjust the pH of the mixed solution, and filter or autoclave to obtain an ophthalmic composition.
[0062] In the method described in this application, the compounded hyaluronate in step 1 has a macromolecular network structure, and the zinc hyaluronate and ectoine or ectoine derivatives in step 2 can be embedded in the macromolecular network structure. The macromolecular network op...
Embodiment 1
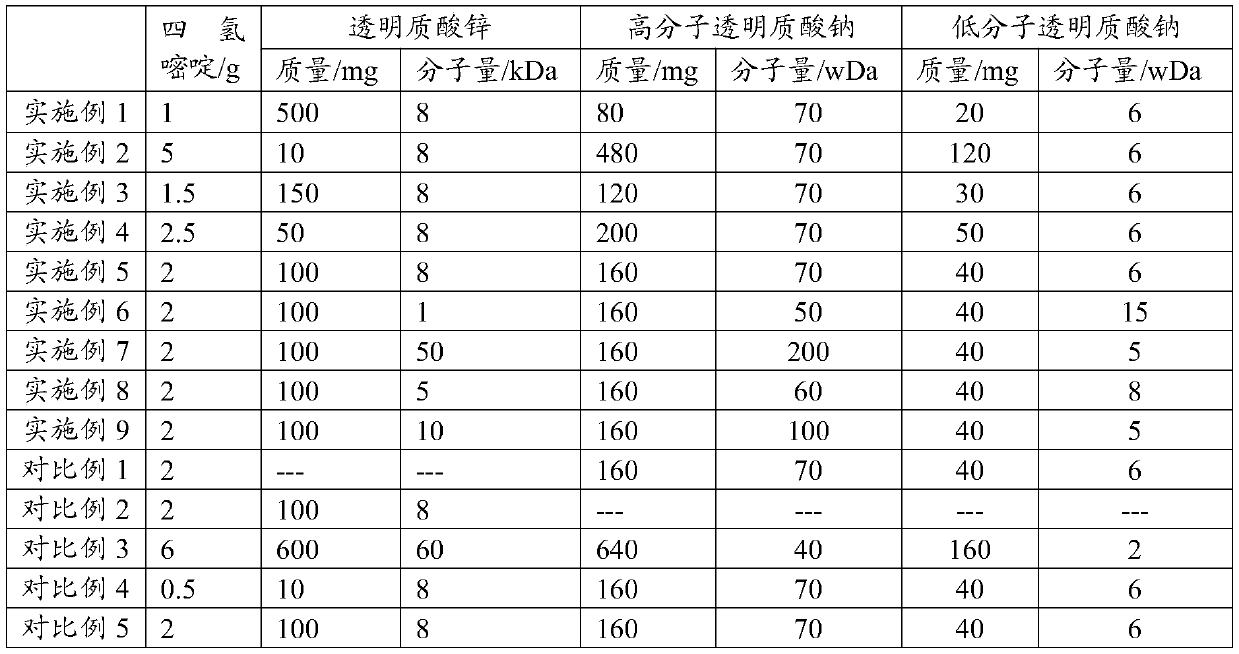
[0112] Take 80 mg of high-molecular-weight sodium hyaluronate with a molecular weight of 70wDa and 20 mg of low-molecular-weight sodium hyaluronate with a molecular weight of 6wDa, dissolve them in water under heating at 60°C, add 2 mg of sodium chloride and stir evenly, and oxidize with hydroxide Adjust the pH to 9.5 with sodium, add dropwise 2 times the volume of 95% ethanol under stirring, the precipitate is completely precipitated, wash the precipitate 4 times with 85% ethanol, and dry in vacuum to obtain a compound sodium hyaluronate powder; the obtained compound hyaluronate Dissolve the sodium bicarbonate powder in water, stir well to dissolve it, add 1g of ectoine and 500mg of zinc hyaluronate (8kDa), stir well, add water for injection to a volume close to 100ml, adjust the pH of the solution to 7.0 with sodium citrate buffer , continue to add water for injection to make the volume to 100ml, filter and sterilize with a filter membrane with a pore size of 0.22 μm to obtai...
Embodiment 5
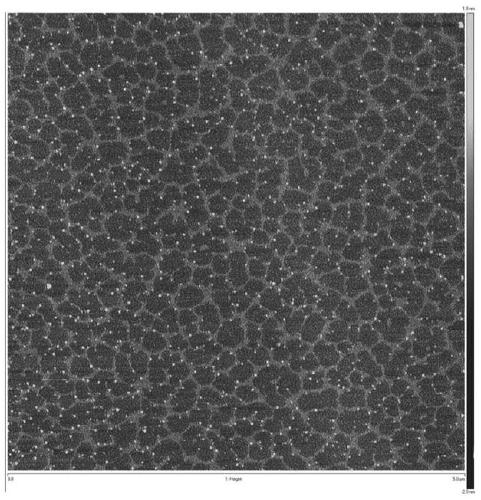
[0122] The compound sodium hyaluronate prepared in Example 5 has a regular network structure after being dissolved in water, and the results are as follows: figure 1 .
[0123] 2. Antibacterial effect
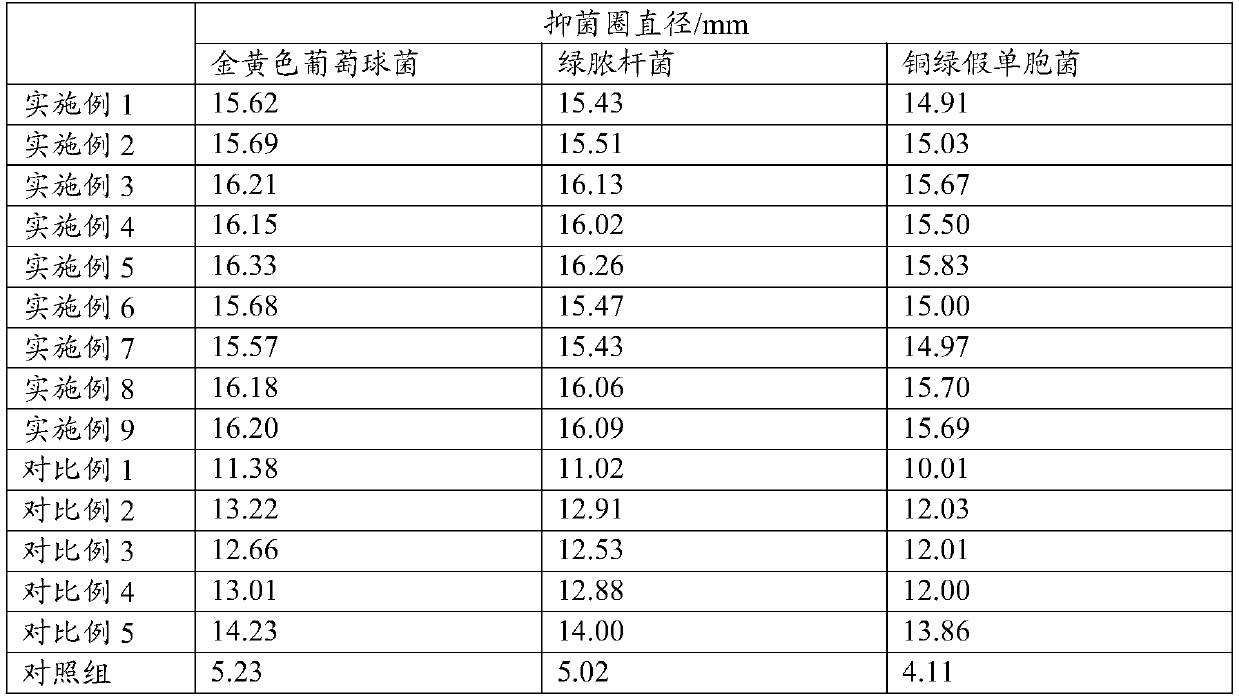
[0124] Take the preserved strains of Staphylococcus aureus, Pseudomonas aeruginosa, and Pseudomonas aeruginosa, and culture them under sterile conditions for 18-24 hours to obtain samples of bacteria in the exponential growth phase, and configure 10 6 The bacterial suspension of CFU / mL is ready for use.
[0125] Take 18 sterilized petri dishes and pour 20mL medium into each petri dish on the ultra-clean workbench. Liquid coated 6 Petri dishes.
[0126] Cut the filter paper sheet into a disc with a diameter of 10mm, put the sterilized filter paper sheet with sterile tweezers on the ultra-clean workbench and put it into the container equipped with the above-mentioned Example 1-Example 9 and Comparative Example 1-Comparative Example 5. Soak in the sterilized petri dish of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com