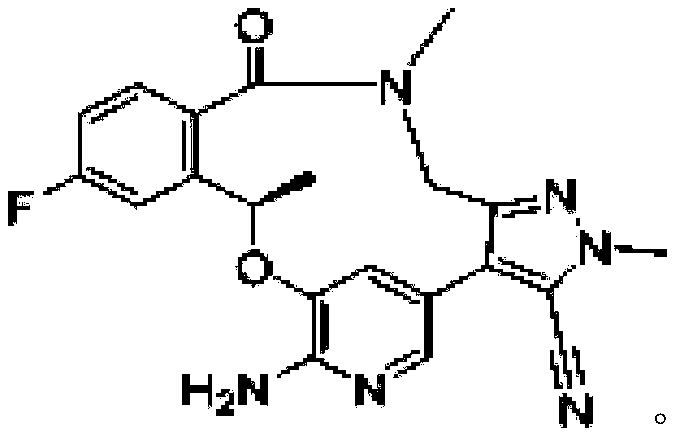
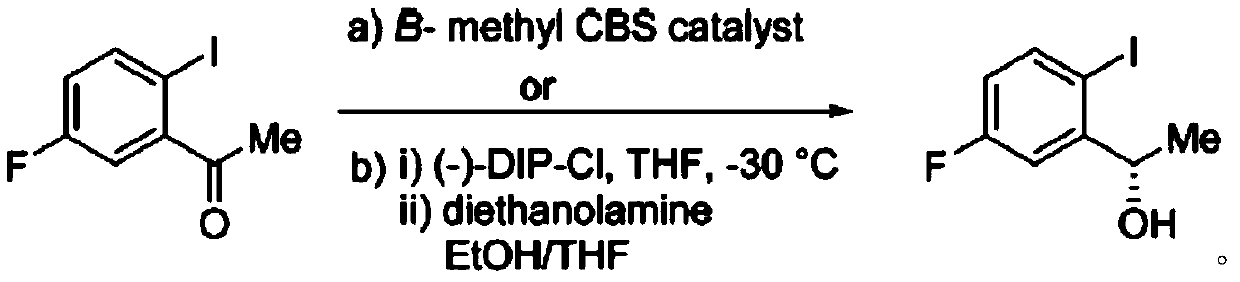
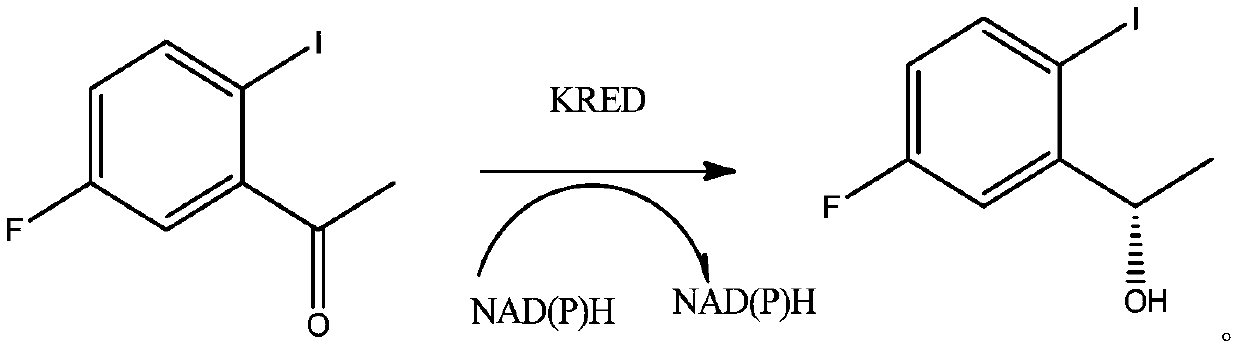
Ketoreductase, nucleic acid, recombinant expression plasmid and strain, and application of ketoreductase in synthesis of Lorlatinib intermediate
A technology for expressing plasmids and reductases, applied in the fields of oxidoreductase, application, recombinant DNA technology, etc., can solve the problems of low yield, unfriendly reaction conditions, etc., achieve high yield, ensure enzyme activity efficiency, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Cloning of the ketoreductase KRED017 gene:
[0044] 1) A ketoreductase was screened out from the enzyme library, numbered KRED017; its DNA sequence is shown in SEQ ID NO:1.
[0045] 2) Synthesize a primer pair F1 (the nucleotide sequence is SEQ ID NO:3) and F2 (the nucleotide sequence is SEQ ID NO:4) according to SEQ ID NO:1. The PCR 20ul system is as follows: 2mmol / L dNTP 2μL, 10×PCR buffer 2μL, PCR high-fidelity enzyme 0.5μL, DNA template obtained in Example 1 1μL, ddH2O 12μL, F1 and F2 each 1μL (5mmol / L). The PCR amplification steps are: ①pre-denaturation at 95°C for 5 min; ②denaturation at 98°C for 15 s; ③annealing at 58°C for 35 s; ④extension at 72°C for 50 s; steps ②~④ were repeated 30 times; The PCR product was purified by agarose electrophoresis, and the target band was recovered with an agarose gel recovery kit to obtain a complete sequence, which could be verified by DNA sequencing.
Embodiment 2
[0047] Expression of the ketoreductase KRED017 gene:
[0048] The PCR product and the pET21a vector described in Example 1 were simultaneously digested with NdeI / XhoI, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel recovery kit; under the action of T4DNA ligase, The target fragment was ligated with the pET21a vector after double digestion to obtain the recombinant expression plasmid pET21a-KRED017.
[0049]Transform the above-mentioned recombinant expression plasmid pET21a-KRED017 into E.coli BL21(DE3) (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) competent cells, spread on LB plates containing 50 μg / mL ampicillin, and culture overnight at 37°C Finally, select positive colonies and inoculate them into 50mL liquid LB medium for cultivation. The composition of liquid LB medium is: peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.2; after culturing for 4 hours, draw 10ml of seeds The solution was transferred t...
Embodiment 3
[0051] Synthetic (S)-1-(2-iodo-5-fluorophenyl)ethanol comprises the following steps:
[0052] 1) Add 50ml of 50mmol / L citric acid buffer solution into a 200mL three-necked flask, the pH of the buffer solution is 6.0, and stir;
[0053] 2) Add 10ml of the crude enzyme lysate described in Example 2, glucose dehydrogenase (GDH) 1000U (purchased from sigama company) and 15g of glucose, add 10g of precursor carbonyl compound 2-iodo-5fluoroacetophenone, Then dilute the volume to 100ml with citric acid buffer, react at 25°C, control the pH with 2mol / L sodium carbonate, and detect the completion of the reaction with TLC after 16 hours of reaction;
[0054] 3) Filter the reaction system to remove protein, extract the aqueous phase and wash the filter cake twice with an equal volume of ethyl acetate (EA);
[0055] 4) The organic phases were combined, and the solvent was recovered under reduced pressure to obtain a crude product, namely (S)-1-(2-iodo-5-fluorophenyl)ethanol.
[0056] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com