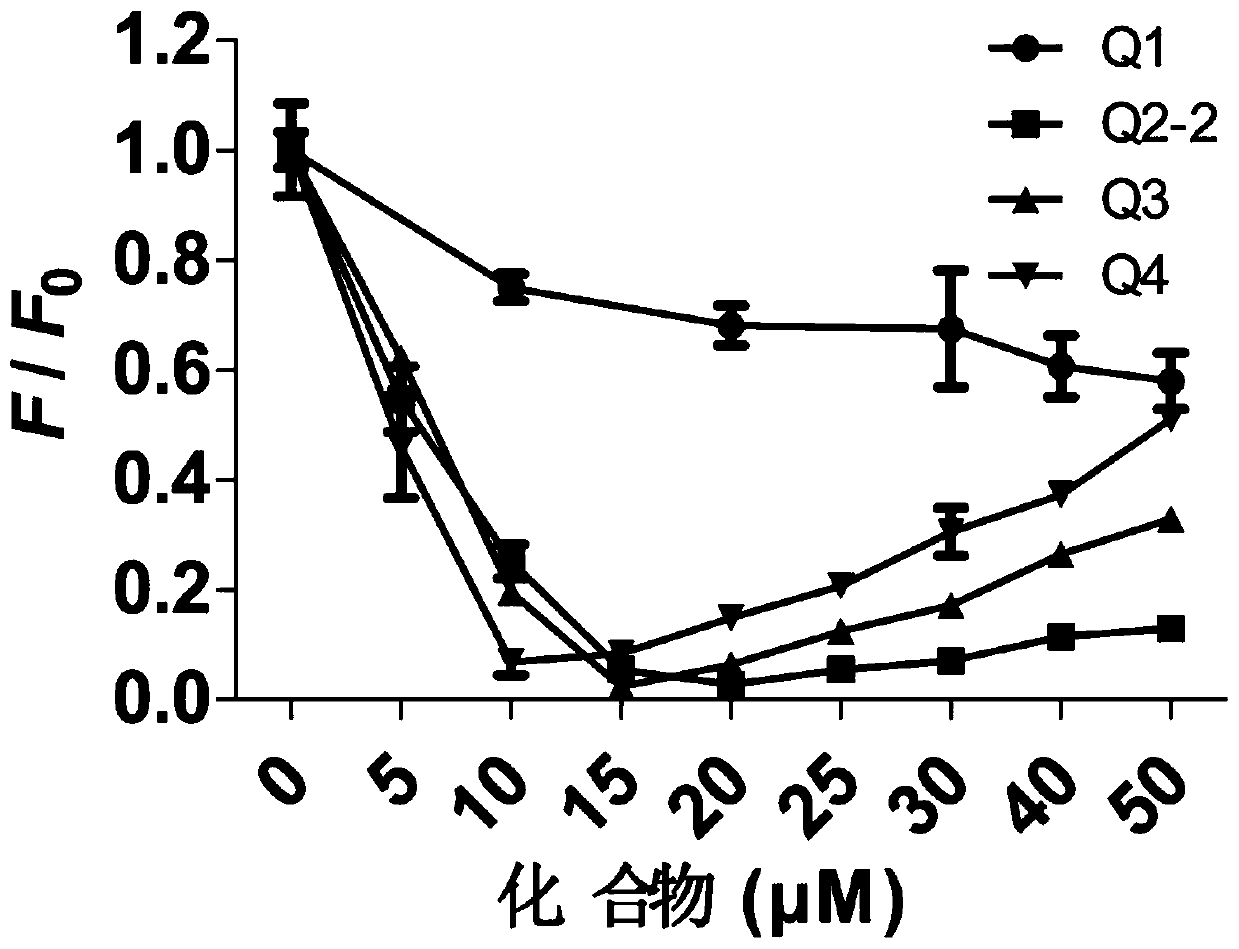
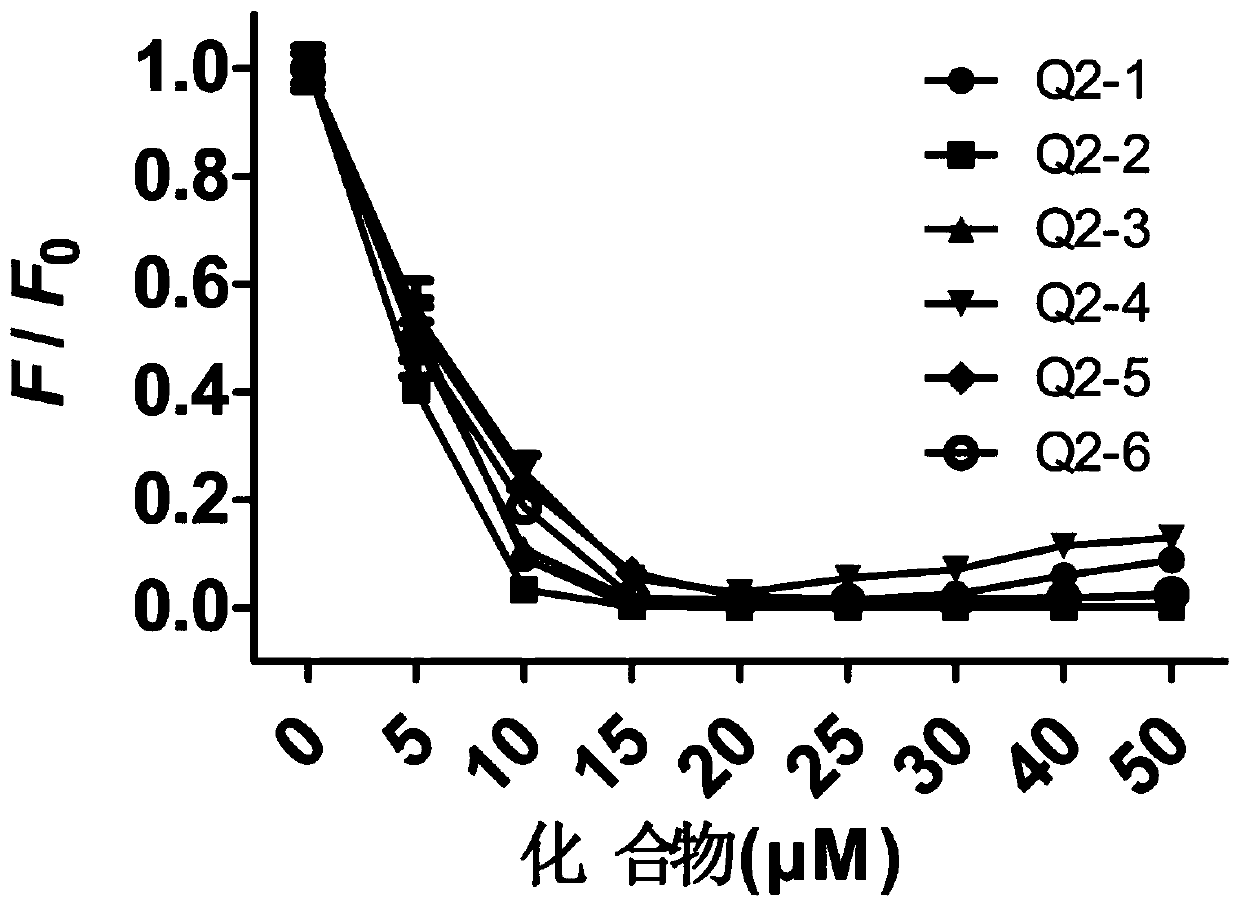
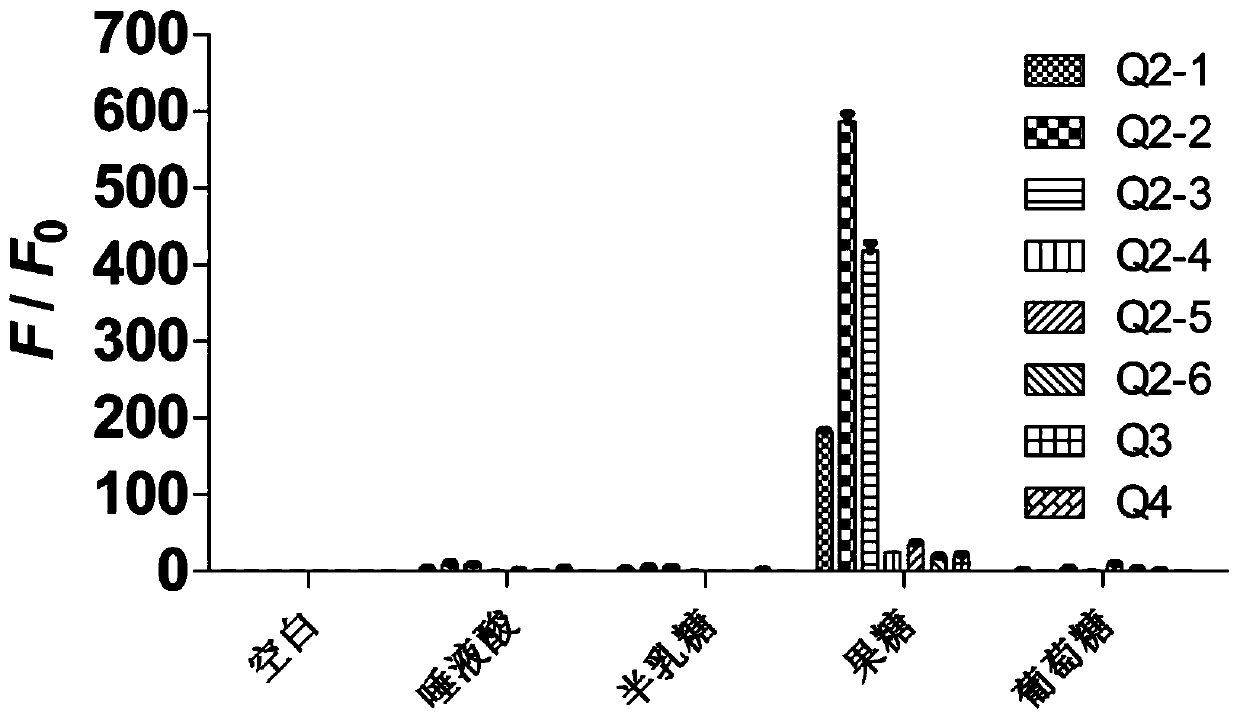
Tetraphenylethylene borate pyridinium salt, preparation method and application thereof, and reagent and method for detecting fructose
A technology of tetrastyrene boronic acid-based pyridinium salt and styrene boronic acid-based pyridinium salt, which can be applied in chemical instruments and methods, measuring devices, compounds containing elements of Group 3/13 of the periodic table, etc. Poor sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The synthetic route of compound Q1 is as follows:
[0073]
[0074] Synthesis of compound 1-1
[0075] N 2 Under protection, benzophenone (100mg, 0.55mmol) was dissolved in 10mL of anhydrous toluene, carbon tetrabromide (364mg, 1.10mmol) and triphenylphosphine (577mg, 2.20mmol) were added sequentially, and refluxed for 4 days. After cooling to room temperature, the reaction solution was suction-filtered. After suction-filtration, liquid column chromatography (petroleum ether) gave Compound 1-1, 126 mg of white needle-like solid, with a yield of 72%.
[0076] Compound 1-1: 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.36-7.29(m,10H).
[0077] Synthesis of Compound 1-2
[0078] N 2 Under protection, compound 1-1 (100 mg, 0.30 mmol) was dissolved in 4 mL of anhydrous toluene, and 4-pyridineboronic acid (109 mg, 0.89 mmol), tetrakis(triphenylphosphine) palladium (104 mg, 0.09 mmol) and 0.5 mL 2N K 2 CO 3 solution, refluxed for 24 hours. Cool to room temperature, extrac...
Embodiment 2
[0084] The synthetic route of compound Q2 is as follows:
[0085]
[0086] Synthesis of compound 2-1
[0087] N 2 Under protection, 4-bromobenzophenone (10.00g, 38.30mmol) was dissolved in 200mL of anhydrous THF, zinc powder (22.53g, 344.70mmol) was added, and TiCl was added dropwise at -20°C 4 (18.94mL, 172.33mmol), refluxed overnight and cooled to room temperature. Adjust the pH to 7 with 1N HCl, evaporate THF under reduced pressure, extract with dichloromethane, wash the organic phase with water and saturated brine, and anhydrous MgSO 4 Dry, filter with suction, concentrate, and separate by column chromatography (petroleum ether) to obtain compound 2-1, 6.80 g of white solid, with a yield of 72%.
[0088] Compound 2-1: m.p.174-176°C; 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.32-7.28(m,4H),7.20-7.15(m,6H),7.06-7.02(m,4H),6.96-6.91(m,4H).
[0089] Synthesis of compound 2-2a
[0090] N 2 Under protection, compound 2-1 (100mg, 0.20mmol) was dissolved in 2mL THF, and 3-py...
Embodiment 3
[0108] The synthetic routes of compounds Q3 and Q4 are as follows:
[0109]
[0110] Synthesis of Compound 3
[0111] N 2 Under protection, 4,4'-dibromobenzophenone (3.50g, 10.29mmol) was dissolved in 40mL of anhydrous THF, zinc powder (3.36g, 25.73mmol) was added, and TiCl was added dropwise at -20°C 4 (3.00mL, 25.73mmol), refluxed overnight and cooled to room temperature. Adjust the pH to 7 with 1N HCl, evaporate THF under reduced pressure, extract with dichloromethane, wash the organic phase with water and saturated brine respectively, and anhydrous MgSO 4 Drying, suction filtration, concentration, and column chromatography (petroleum ether) gave Compound 3, 3.04 g of a white solid, with a yield of 90%.
[0112] Synthesis of Compound 3-1
[0113] N 2 Under protection, compound 3 (300mg, 0.46mmol) was dissolved in 6mL THF, and 4-pyridineboronic acid (342mg, 2.78mmol), tetrakis(triphenylphosphine) palladium (924mg, 0.8mmol) and 3mL 2N K 2 CO 3 solution, refluxed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com