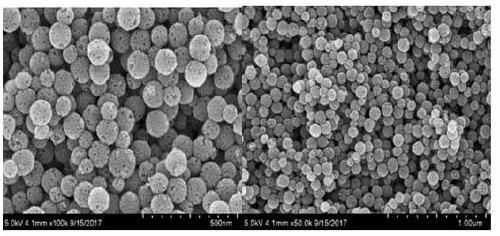
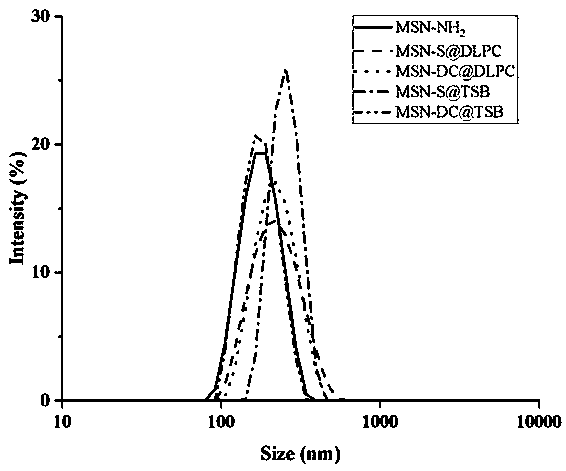
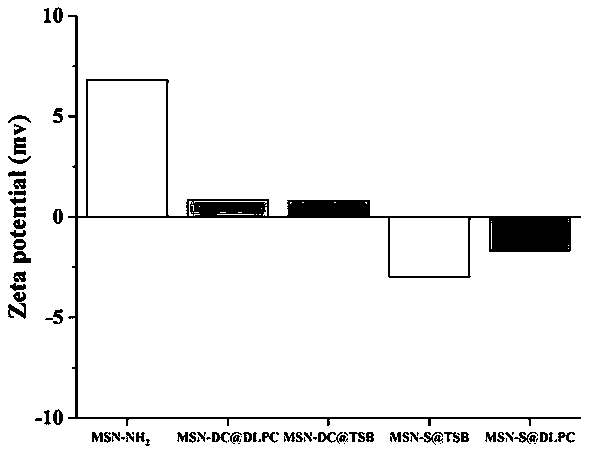
Oral administration system capable of promoting trans-mucus penetration of protein drug and preparation of oral administration system
A protein drug and drug delivery system technology, applied in the field of oral drug delivery system and its preparation, can solve the problems of weakening, complicated production process, induration and inflammation of injection site
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0053] Example 1 Preparation of Hydrophobic Modified Mesoporous Silica
[0054] sample 1
[0055] Weigh 0.5g CTAB and dissolve it in 160mL double-distilled water, stir in a 60°C water bath at a speed of 600rpm for 0.5h to fully dissolve, add 70mL n-octane dropwise to the above clear solution, and connect to a condensing reflux device , and continued to stir for 2h to obtain a uniform emulsion. Slowly add 18 mL of styrene monomer dropwise to the emulsion. Under nitrogen protection, 0.11 g of lysine, 5.0 mL of tetraethyl orthosilicate, and 0.181 g of azobisisobutylamidine hydrochloride were sequentially added to the reaction solution. After reacting for 3 hours, the suspension was naturally cooled, left to stand for about 10 hours, an equal volume of absolute ethanol was added, centrifuged at 10000 rpm for 8 minutes, and the precipitate was washed 3 times with absolute ethanol. After the precipitate was dried at 60°C for 12h, it was calcined at 550°C for 6h to remove the pore...
example 2
[0059] Example 2 Preparation of self-assembled nanoparticles loaded with insulin
[0060] sample 1
[0061] Weigh 3 mg of the carrier prepared in sample 1 in Example 1, 3 mg of insulin and 3 mg of dodecyl betaine and dissolve them in 0.2 mL of DMSO, slowly add the above suspension into 5 ml of distilled water, and centrifuge at 10,000 rpm for 8 min to obtain the carrier Drug System (MSN-S@TSB).
[0062] sample 2
[0063] Weighed 3 mg of the carrier prepared in sample 2 in Example 1, 3 mg of insulin and 3 mg of dilauroyl lecithin were dissolved in 0.2 mL of DMSO, slowly added the suspension to 5 ml of distilled water, and centrifuged at 10,000 rpm for 8 min to obtain the carrier Drug System (MSN-DC@TSB).
[0064] sample 3
[0065] Weighed 3 mg of the carrier prepared in sample 1 in Example 1, 3 mg of insulin and 3 mg of dilauroyl lecithin were dissolved in 0.2 mL of DMSO, slowly added the suspension to 5 ml of distilled water, and centrifuged at 10,000 rpm for 8 min to obta...
example 3
[0069] Example 3 Cell Uptake Experiment
[0070] Caco-2 cells were treated with 1×10 5 / mL concentration was cultured in a 12-well plate, and the cells were cultured until the confluence reached 90%. After the cells were washed with PBS, the vector was dispersed into a serum-free medium and incubated with Caco-2 cells at 37°C for 4 hours. After the incubation, the nanoparticle dispersion was discarded, and the cells were washed 3 times with ice-cold PBS to terminate cell uptake. Add 0.25% trypsin to digest cells, suspend with 0.3mL PBS, use blank cells as negative control, set 3 wells for each sample, collect 10,000 cells each, measure the average fluorescence intensity, MSN-S@DLPC, MSN-S@ Compared with MSN-NH, TSB, MSN-DC@DLPC, and MSN-DC@TSB respectively 2 Increased to 1.2, 2, 20 and 13 times (average fluorescence intensity as attached Figure 4 ). This result shows that the drug delivery system prepared by the present invention can significantly improve the uptake of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com