Synthesis method of cycloalkane compounds
A synthesis method and technology of cycloalkane, which is applied in the field of synthesis of cycloalkane compounds, can solve problems such as easy agglomeration, harsh requirements for raw material impurities, harsh operating conditions, etc., and achieve suitable storage and transportation, good catalytic performance, and good repeatability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The present invention provides a kind of synthetic method of cycloalkane compound, comprising:
[0059] A carbon-coated nickel nanocomposite material containing alkaline earth metal is used as a catalyst, and after the catalyst is mixed with an aromatic hydrocarbon compound in a solvent, a hydrogenation reduction reaction is carried out under a hydrogen atmosphere; the chemical reaction equation is exemplified as follows:
[0060]
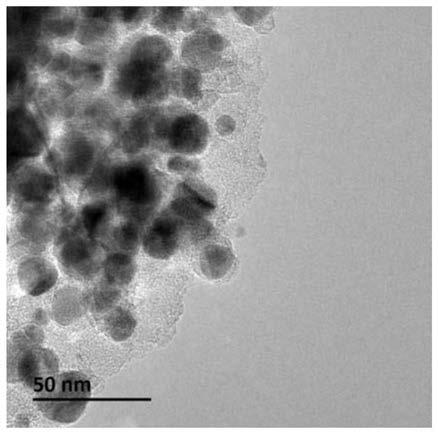
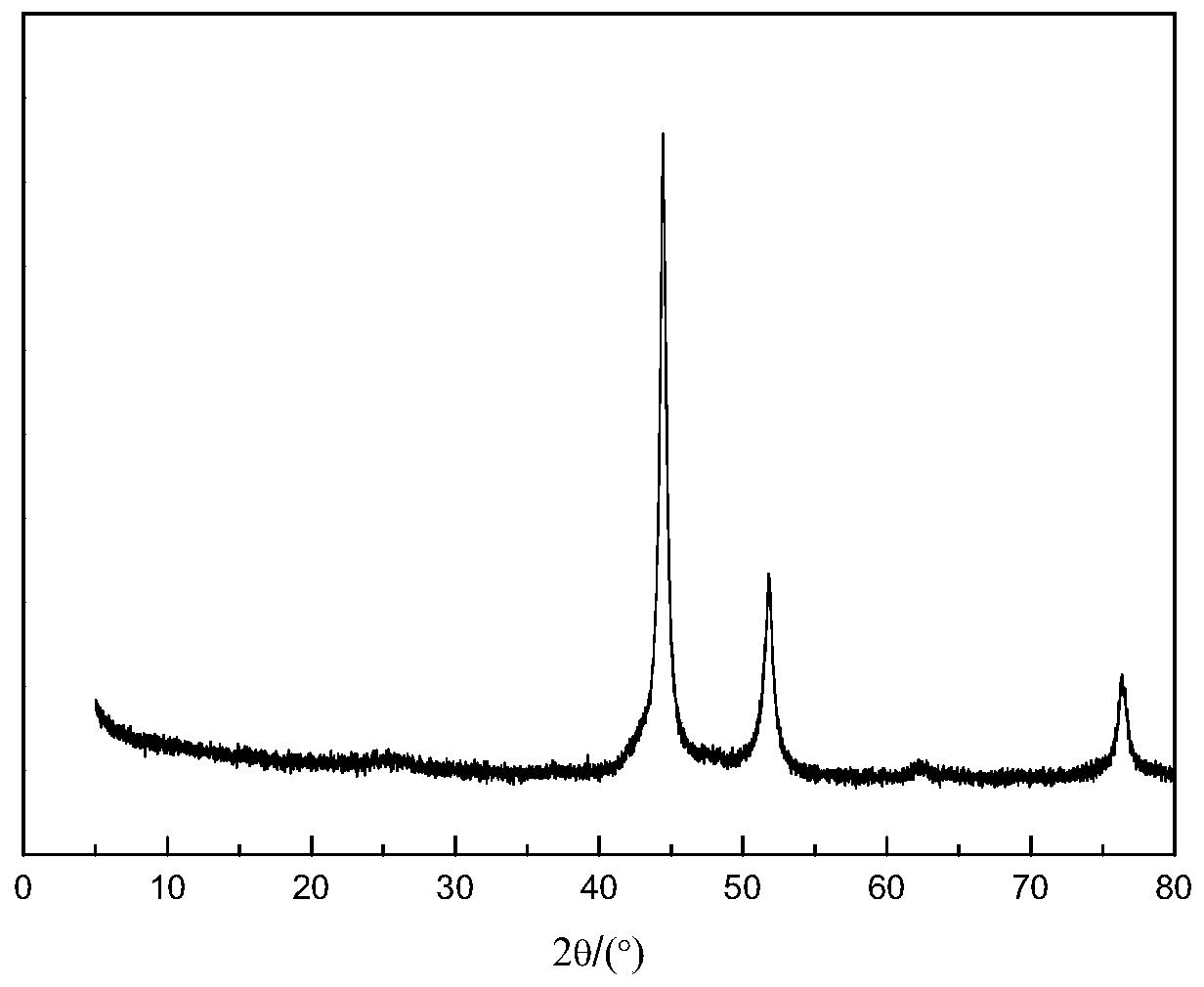
[0061] Wherein, the nanocomposite material has a core-shell structure with a shell and an inner core, the shell is a graphitized carbon layer containing alkaline earth metal and oxygen, and the inner core is nickel nanoparticles.
[0062] In some embodiments, the aromatic ring of the aromatic hydrocarbon compound also contains a substituent R, and the substituent is selected from C 1-20 One or more of the alkyl, cycloalkyl and aryl groups. The above-mentioned substitutions may be mono-substituted or poly-substituted, including but not li...
preparation example 1
[0103] (1) Weigh 10g of nickel acetate and 10g of citric acid into a beaker containing 30mL of deionized water, stir at 70°C to obtain a homogeneous solution, continue heating and evaporate to dryness, and obtain a solid precursor.
[0104] (2) Place the solid precursor obtained in step (1) in a porcelain boat, then place the porcelain boat in the constant temperature zone of the tube furnace, feed nitrogen gas with a flow rate of 100mL / min, and set the temperature at a rate of 5°C / min. Raise the temperature to 650°C, stop the heating after keeping the temperature for 2 hours, and cool to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel material.
[0105] (3) Weigh 2g of the material obtained in step (2), add 4ml of an aqueous solution containing 0.1528g of magnesium nitrate, soak at room temperature for 24h, and then dry the product at 120°C.
[0106] (4) The dried material obtained in step (3) is placed in a porcelain boat, and then the porcelain...
preparation example 2
[0112] (1) Weigh 10g of nickel acetate and 20g of citric acid into a beaker containing 50mL of deionized water, stir at 80°C to obtain a homogeneous solution, continue heating and evaporate to dryness, and obtain a solid precursor.
[0113] (2) The solid obtained in step (1) is placed in a porcelain boat, and then the porcelain boat is placed in the constant temperature zone of the tube furnace, and the nitrogen gas with a flow rate of 150mL / min is fed into it, and the temperature is raised to 600°C, keep the temperature constant for 2 hours, stop heating, and cool to room temperature under a nitrogen atmosphere to obtain a carbon-coated nickel material.
[0114] (3) Weigh 2 g of the carbon-coated nickel material obtained in step (2), add 4 mL of an aqueous magnesium nitrate solution containing 0.8589 g, soak at room temperature for 24 h, and then dry the product at 120° C.
[0115] (4) The dried material obtained in step (3) is placed in a porcelain boat, and then the porcela...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com