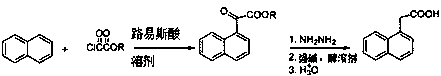
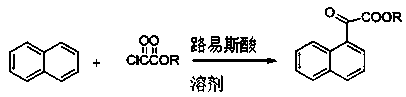
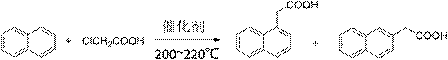Preparation method of alpha-naphthylacetic acid
A technology of naphthalene acetic acid and naphthalene ethyl ketone ester, which is applied in the direction of carboxylate preparation, carboxylate preparation, carboxylate preparation, etc., can solve the problems of high environmental protection costs, expensive α-naphthylacetone, etc., and achieve production costs Low, easy to purify, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In the reaction flask, add 120 mL (1.5 mol) of 1,2-dichloroethane, 14.8 g (0.111 mol) of anhydrous aluminum trichloride and 12.8 g (0.1 mol) of naphthalene; stir well and heat to 45 °C , add 12.3 mL (0.115 mol) monoethyl oxalyl chloride; react for 4 hours, after the reaction, pour the reaction mixture into 100 mL cold water, stir well and let it stand, separate the organic layer; wash the organic layer with 30 mL 5% hydrochloric acid After pickling, washing with water, and drying with anhydrous sodium sulfate, it was concentrated to obtain an oily product (product 1); product 1 was directly used in the next reaction without purification.
[0038] Add the above product 1 into the reaction flask, add 110 mL (1.98 mol) of ethylene glycol and 12 mL (0.32 mol) of hydrazine hydrate (85%); slowly raise the temperature to 130 °C under stirring, and react for 2 hours; then cool the reaction solution to Below 80 °C, add 26.9 g (0.48 mol) of potassium hydroxide, then heat the reac...
Embodiment 2
[0040]In the reaction flask, add 120 mL (1.24 mol) of carbon tetrachloride, 20.4 g (0.15 mol) of anhydrous zinc chloride and 12.8 g (0.1 mol) of naphthalene; after stirring evenly, heat to 55 °C, add 11.0 mL ( 0.12 mol) monomethyl oxalyl chloride; react for 4 hours, after the reaction, pour the reaction mixture into 100 mL of cold water, stir well and let stand, and separate several layers; the organic layer is pickled with 30 mL of 5% hydrochloric acid, washed with water, After drying over sodium sulfate, it was concentrated to obtain an oil (product 1); the product 1 was directly used in the next reaction without purification.
[0041] Add the above product 1 into the reaction flask, add 110 mL (1.36 mol) of 1,2-propanediol and 6.5 mL (0.21 mol) of anhydrous hydrazine; slowly raise the temperature to 130 °C under stirring, and react for 2 hours; then cool the reaction liquid to 80 Below ℃, add 12g (0.3 mol) sodium hydroxide, then heat the reaction mixture to 180 ℃ for 4 hour...
Embodiment 3
[0043] In the reaction flask, add 95 mL (1.48 mol) of dichloromethane, 35 g (0.134 mol) of anhydrous tin chloride and 12.8 g (0.1 mol) of naphthalene; mol) ethyl oxalyl chloride; reacted for 4.5 hours, poured the reaction mixture into 100 mL of cold water after the reaction was completed, stirred thoroughly and allowed to stand, separated into several layers; the organic layer was pickled with 30 mL of 5% hydrochloric acid, washed with water, After drying over sodium sulfate, it was concentrated to obtain an oil (product 1); the product 1 was directly used in the next reaction without purification.
[0044] Add the above product 1 into the reaction flask, add 135 mL (1.5 mol) of 1,4-butanediol and 18 mL (0.48 mol) of hydrazine hydrate (85%); slowly raise the temperature to 140 °C under stirring, and react for 2 hours; then Cool the reaction liquid to below 80°C, add 26.9 g (0.48 mol) of potassium hydroxide, then raise the temperature of the reaction mixture to 190°C for 4 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com