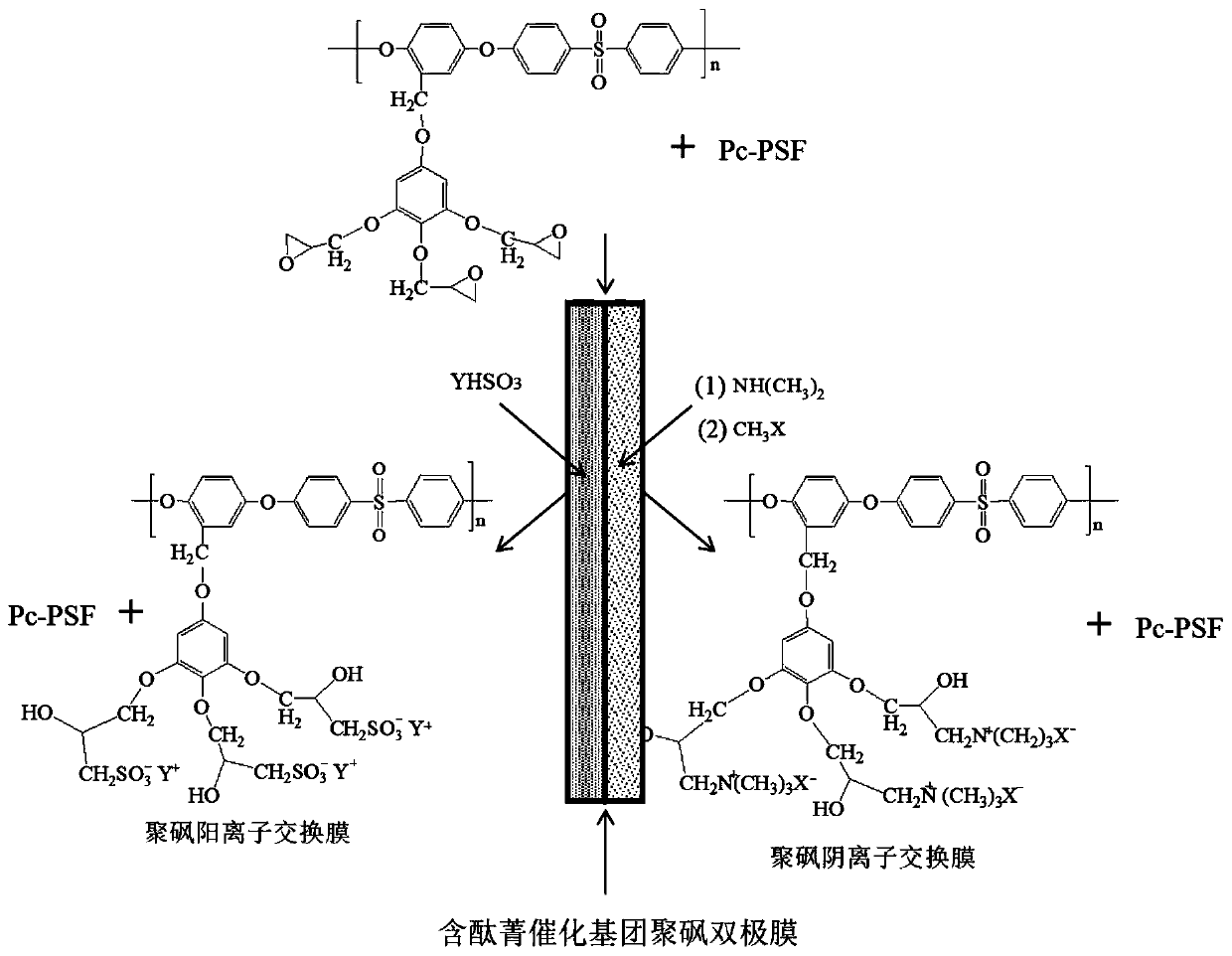
Preparation method of monolithic polysulfone bipolar membrane with side group bonded with phthalocyanine catalytic group
A technology for synthesizing phthalocyanine catalytic groups and bipolar membranes is applied in the field of preparation of monolithic polysulfone bipolar membranes with side groups bonded to phthalocyanine catalytic groups, and can solve the chemical stability and thermal stability of functional groups. It is not high, the scope of use is limited, the utilization rate of monomer is low, etc., to achieve good economic benefits and promotion value, improve stability and service life, and save the effect of film forming process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
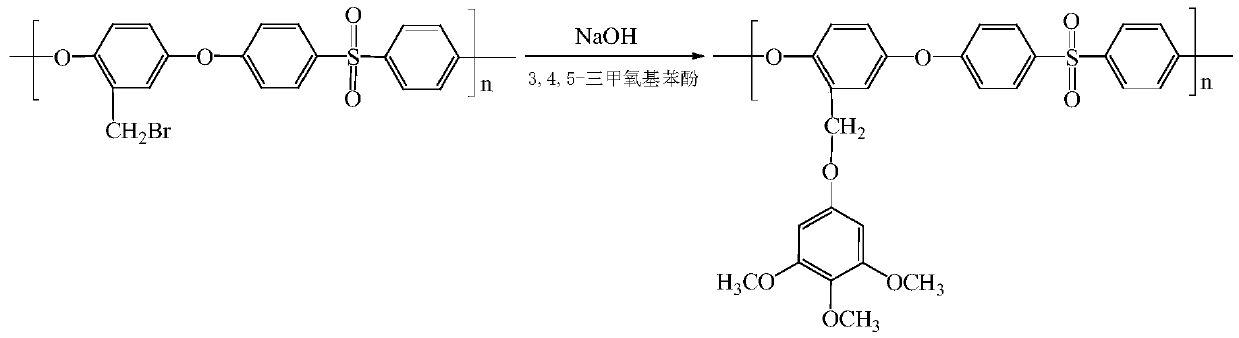
[0044] (1) Add 5.0 g of bromomethylated polysulfone into a three-necked flask, dissolve with 60 mL of N-methylpyrrolidone, and set aside. Weigh again 2.39g 3,4,5-trimethoxyphenol and 0.52g NaOH, dissolve with 30mL N-methylpyrrolidone, stir at room temperature for 0.5h, add dropwise into bromomethylated polysulfone solution, and stir at room temperature 10h, after the reaction is over, use a mixed solvent precipitant with a volume ratio of water and ethanol of 1:1 to precipitate the product. After filtering, the precipitate is rinsed with ethanol several times, and the precipitate is soaked in deionized water. After 24 hours, filter and precipitate The product was vacuum-dried at 70°C to prepare a methoxy-containing polysulfone polymer.
[0045]
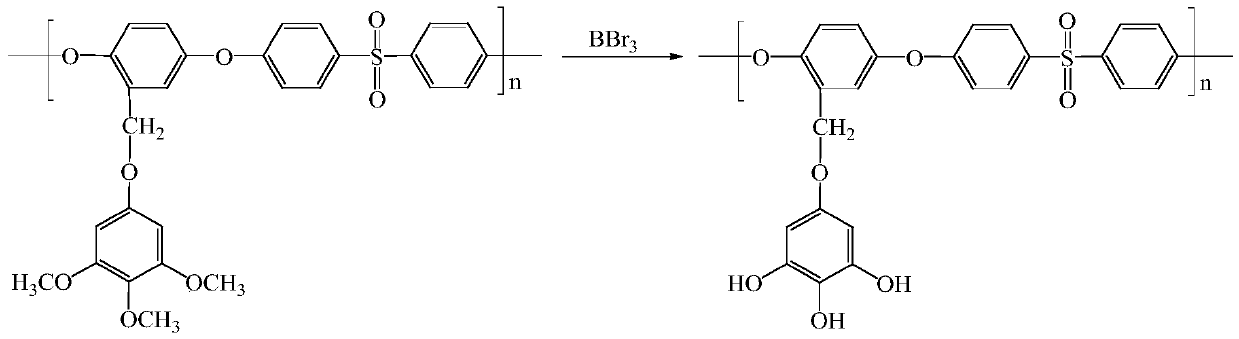
[0046] (2) Weigh 5.0 g of methoxy-containing polysulfone polymer into a 250 mL three-necked flask, install a constant-pressure dropping funnel, and protect it with a nitrogen balloon after vacuuming. Add 120mL of pre-dried dichlor...
Embodiment 2
[0058] (1) Synthesis of water catalyst in the middle layer of bipolar membrane-side chain bonded phthalocyanine substituent Pc-PSF polymer: In a 100mL three-necked flask, add 5.0g bromomethylated polysulfone polymer and 60mL NMP, and wait for copolymerization After the substance is fully dissolved, 20mL NMP solution dissolved with 6.6g hydroxyphthalocyanine iron and 1.0g NaHCO 3 , put them into a three-necked flask, raise the temperature to 50°C under the protection of nitrogen, and react at a constant temperature for 5 hours. After the reaction, immediately cool the system to room temperature with an ice-water bath, and use a mixture of distilled water and methanol as a precipitant to precipitate the product polymerization The product was washed with the mixed solution several times, and dried in vacuum to obtain a polymer (Pc-PSF) with phthalocyanine (Pc) bonded to the side chain.
[0059]
[0060] (2) Add 5.0 g of bromomethylated polysulfone into a three-necked flask, di...
Embodiment 3
[0071] (1) Synthesis of side group-bonded phthalocyanine polysulfone polymer (Pc-PSF) bipolar membrane interlayer water dissociation catalyst: In a 100mL three-neck flask, add 5.0g bromomethylated polysulfone polymer and 60mL NMP, After the copolymer is fully dissolved, dissolve 20mL of NMP solution with 6.6g of hydroxyphthalocyanine manganese and 1.0g of NaHCO 3 , put them into a three-necked flask, raise the temperature to 50°C under the protection of nitrogen, and react at a constant temperature for 5 hours. After the reaction, immediately cool the system to room temperature with an ice-water bath, and use a mixture of distilled water and methanol as a precipitant to precipitate the product polymerization The product was washed with the mixed solution several times, and dried in vacuum to obtain a polymer (Pc-PSF) with phthalocyanine (Pc) bonded to the side chain.
[0072]
[0073] (2) Add 5.0 g of bromomethylated polysulfone into a three-necked flask, dissolve with 60 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com