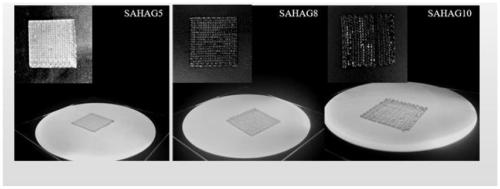
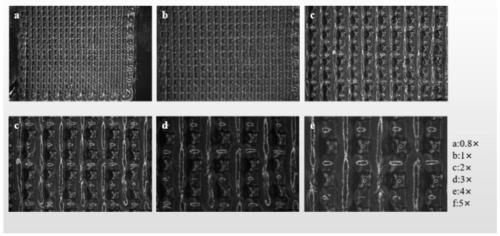
Biological 3d printed active biological membrane for improving AMIC technology cartilage repair and preparation method thereof
An active biofilm and cartilage repair technology, applied in tissue regeneration, medical science, prosthesis, etc., can solve the problems of incompatibility of allogeneic bone reconstruction, different size, shape and curvature, abnormal intra-articular stress, etc., and achieve the ability to significantly promote cartilage. , good histocompatibility and biodegradability, and the effect of promoting cartilage regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1) Printing material preparation
[0061] 1.1) Aseptic treatment: gelatin, sodium alginate, hyaluronic acid, and calcium chloride powder were sterilized by ultraviolet light for 24 hours, and a magnetic heating stirrer was placed on an ultra-clean bench to prepare a hydrogel pre-preparation solution. All other operations are performed on a clean bench.
[0062] 1.2) Preparation of gelatin solution: Heat 2.5g of gelatin with 20ml of deionized water in a 48°C water bath, stir with a constant temperature magnetic stirrer at a speed of 300r / min, and let it fully dissolve to prepare a gelatin solution.
[0063] 1.3) Preparation of sodium alginate / gelatin solution: Add 20ml of deionized water and 1.25g of sodium alginate to the gelatin solution, heat in a water bath at 48°C with a rotation speed of 300r / min, and fully dissolve it into a sodium alginate / gelatin solution.
[0064] 1.4) Preparation of sodium alginate / gelatin / hyaluronic acid mixed solution: add 10ml of deionized...
Embodiment 2
[0073] 1) Printing material preparation
[0074] 1.1) Aseptic treatment: gelatin, sodium alginate, hyaluronic acid, and calcium chloride powder were sterilized by ultraviolet light for 24 hours, and a magnetic heating stirrer was placed on an ultra-clean bench to prepare a hydrogel pre-preparation solution. All other operations are performed on a clean bench.
[0075] 1.2) Preparation of gelatin solution: Heat 4g of gelatin in a 48°C water bath with 20ml of deionized water, stir with a constant temperature magnetic stirrer at a speed of 300r / min, and allow it to fully dissolve to prepare a gelatin solution.
[0076] 1.3) Preparation of sodium alginate / gelatin solution: Add 20ml of deionized water and 1.25g of sodium alginate to the gelatin solution, heat in a water bath at 48°C with a rotation speed of 300r / min, and fully dissolve it into a sodium alginate / gelatin solution.
[0077] 1.4) Preparation of sodium alginate / gelatin / hyaluronic acid mixed solution: add 10ml of deionize...
Embodiment 3
[0086] 1) Printing material preparation
[0087] 1.1) Aseptic treatment: gelatin, sodium alginate, hyaluronic acid, and calcium chloride powder were sterilized by ultraviolet light for 24 hours, and a magnetic heating stirrer was placed on an ultra-clean bench to prepare a hydrogel pre-preparation solution. All other operations are performed on a clean bench.
[0088] 1.2) Preparation of gelatin solution: Heat 5g of gelatin with 20ml of deionized water in a water bath at 48°C, stir with a constant temperature magnetic stirrer at a speed of 300r / min, and allow it to fully dissolve to prepare a gelatin solution.
[0089] 1.3) Preparation of sodium alginate / gelatin solution: Add 20ml of deionized water and 1.25g of sodium alginate to the gelatin solution, heat in a water bath at 48°C with a rotation speed of 300r / min, and fully dissolve it into a sodium alginate / gelatin solution.
[0090] 1.4) Preparation of sodium alginate / gelatin / hyaluronic acid mixed solution: add 10ml of dei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com