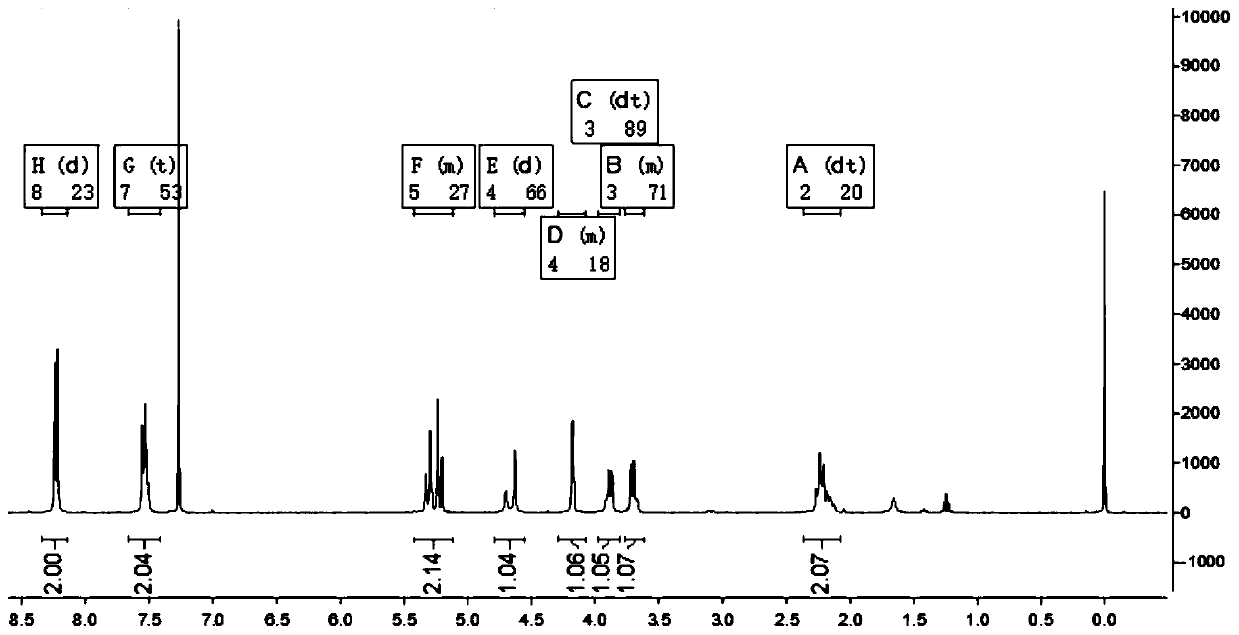
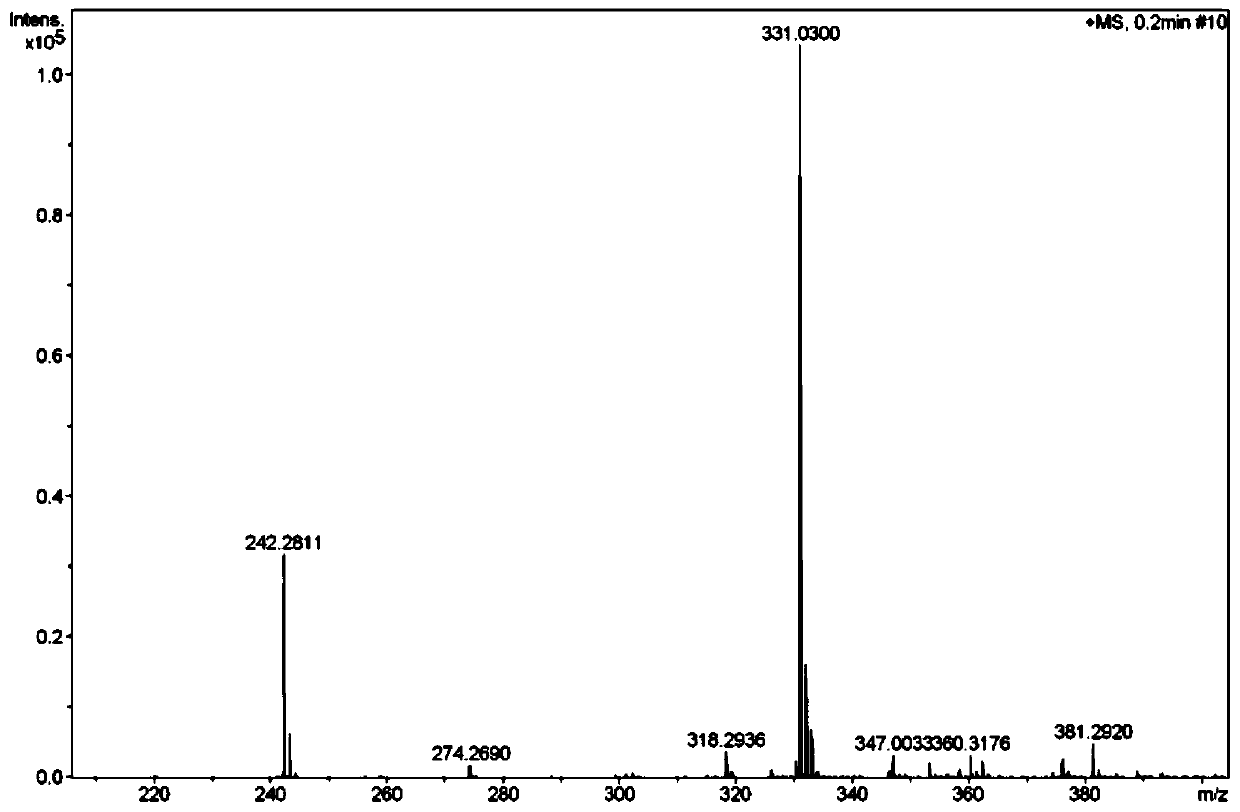
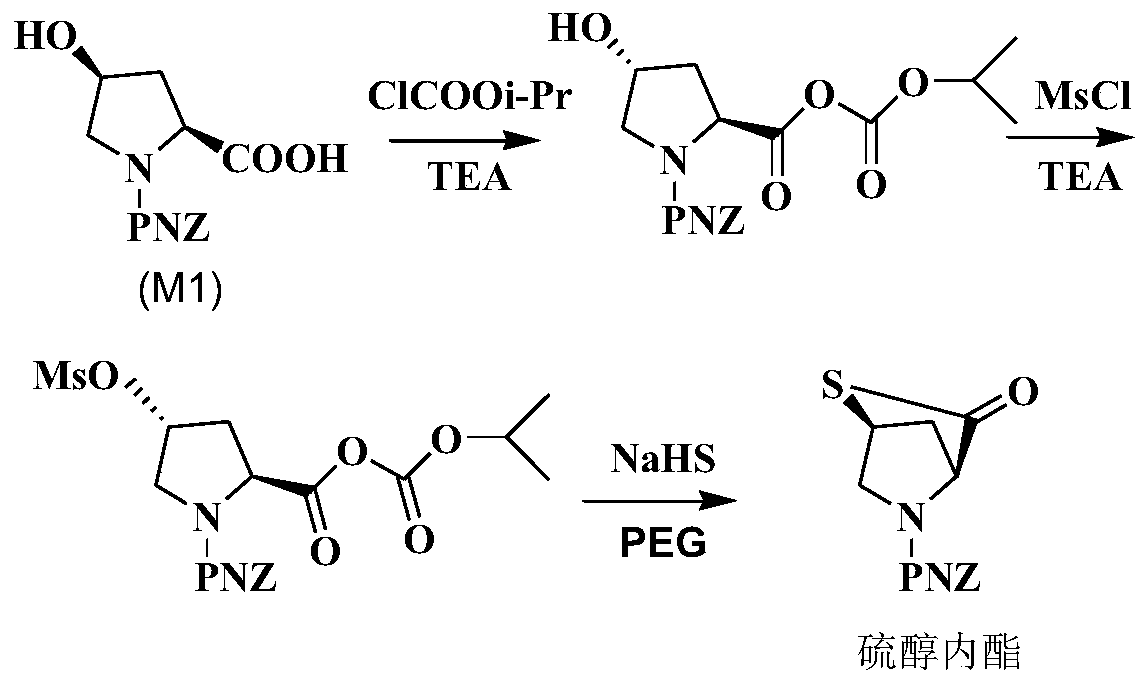
Method for synthesizing meropenem side chain intermediate thiol lactone by using sodium hydrosulfide
A meropenem side chain and sodium hydrosulfide technology, applied in the field of chemical substance preparation, can solve problems affecting the health and safety of operators, increase the cost of personnel and equipment, and unfavorable green production of enterprises, so as to achieve healthy work and easy The effect of mild storage and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add 1.8 g of M1 (ie, [(2S,4R)-2-carboxy-1-(4-nitrobenzyloxycarbonyl)pyrrolidine)] to 32 mL of dichloromethane, keep the liquid temperature at -16°C, First add 0.71g isopropyl chloroformate dropwise, then add 0.76g TEA dropwise, and react for 15 minutes;
[0024] 2) Add 0.86g MsCl dropwise at -16°C, then add 0.70g TEA dropwise, and react for 26min;
[0025] 3) Dissolve 1.81g NaHS, 0.24g PEG-600 and 1.74g water into a solution, add this solution into the reaction mixture solution at -16°C, raise the temperature from -16°C to 0°C, and keep the heating time for 25min; , Add 8 mL of deionized water at 0°C for liquid separation. The organic phase was separated, and the temperature of the organic phase was raised to 40° C. for reflux reaction. The reflux reaction time was 2.5 hours. After the reaction was completed, a thiol lactone solution was obtained;
Embodiment 2
[0028] 1) Add 1.8g M1 to 33mL dichloromethane, keep the liquid temperature at -15°C, add 0.71g isopropyl chloroformate dropwise, then add 0.77g TEA dropwise, and react for 14min;
[0029] 2) Add 0.85g MsCl dropwise at -15°C, then add 0.71g TEA dropwise, and react for 25min;
[0030] 3) Dissolve 1.67g NaHS, 0.25g PEG-2000 and 1.74g water into a solution, add this solution into the reaction solution at -15°C, raise the temperature from -15°C to 0°C, and keep the heating time for 26min; after the heating is completed, Add 9 mL of deionized water at 0°C for liquid separation, separate the organic phase, raise the temperature of the organic phase to 39°C for reflux reaction, and reflux for 3 hours. After the reaction is completed, a thiol lactone solution is obtained;
[0031] 4) Post-treat the obtained thiol lactone solution, add 6% potassium carbonate solution at 1°C to adjust to pH=8, separate oil phase 1, and continue to adjust oil phase 1 to 1 at 1°C with 19% hydrochloric acid...
Embodiment 3
[0033] 1) Add 1.8g M1 to 33.5mL dichloromethane, keep the liquid temperature at -16°C, first add 0.72g isopropyl chloroformate dropwise, then add 0.77g TEA dropwise, and react for 12 minutes;
[0034] 2) Add 0.86g MsCl dropwise at -16°C, then add 0.72g TEA dropwise, and react for 26min;
[0035] 3) Dissolve 1.95g NaHS, 0.26g PEG-600 and 1.74g water into a solution, add this solution into the reaction solution at -16°C, raise the temperature from -16°C to 0°C, and keep the heating time for 25min; after the heating is completed, Add 10 mL of deionized water at 0°C for liquid separation, separate the organic phase, raise the temperature of the organic phase to 38°C for reflux reaction, and reflux for 2.8 hours. After the reaction is completed, a thiol lactone solution is obtained;
[0036] 4) Post-treat the obtained thiol lactone solution, add 5% potassium carbonate solution at 0°C to adjust to pH=8.5, separate the oil phase 1, and continue to adjust the oil phase 1 to 0 with 17%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com