Preparation method of cyromazine
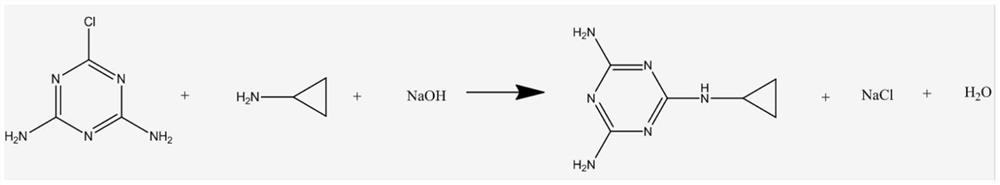
A technology of cyromazine and diamino, which is applied in the field of preparation of veterinary medicine and pharmaceutical raw material drug cyromazine, and can solve the safety hazards and disadvantages of 2-cyclopropylamino-4,6-dichloro-S-triazine Safe production and environmental protection, shortening the response time and other issues, to achieve the effect of solving the contradiction between economic production and environmental protection, strong practical production technology, and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
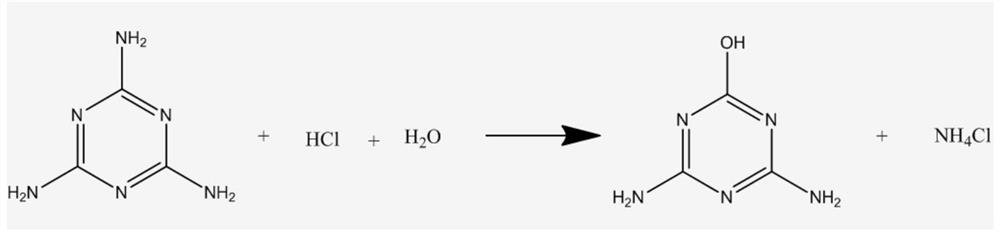
[0024] Take 20.05g of melamine, add it to 100.20g of hydrochloric acid aqueous solution with a pH value of 4, start stirring, and raise the temperature to 100°C. During the reaction, 1M hydrochloric acid is continuously added to maintain the pH value, and the reaction is carried out for 7 hours. Then add 1M sodium hydroxide to adjust the reaction system to a pH value of about 7.0, then cool down to 5°C, filter, and dry to obtain 20.23g of solid, the main component of which is 4,6-diamino-2-hydroxy-1,3 ,5-triazine with a purity of 96.13%.
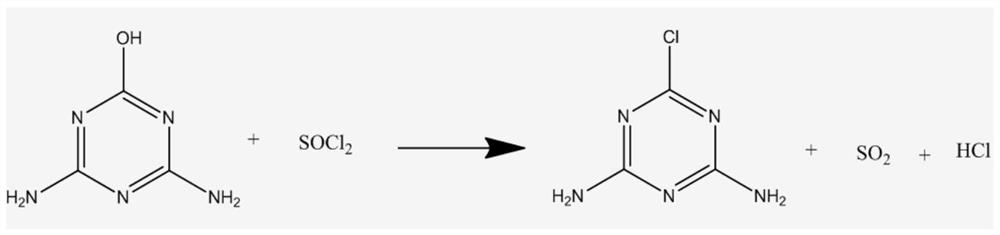
[0025] All the products obtained above were added to 80.65g of toluene, and the temperature of the system was lowered to 5°C. Within 0.5h, 21.87g of thionyl chloride was added dropwise, and the temperature was controlled at 5-10°C. After the dropwise addition was completed, the temperature was raised to 65° C. and kept for 30 minutes. During the chlorination reaction process, acid gas will be released, so attention should be paid to tail ga...
Embodiment 2
[0028] Take 20.13g of melamine, add it to 100.05g of hydrochloric acid aqueous solution with a pH value of 3.5, start stirring, and raise the temperature to 100°C. During the reaction, 1M hydrochloric acid is continuously added to maintain the pH value, and the reaction is carried out for 7 hours. Then add 1M sodium hydroxide to adjust the reaction system to a pH value of about 7.0, then cool down to 5°C, filter, and dry to obtain 20.30 g of solids, the main component of which is 4,6-diamino-2-hydroxy-1,3 ,5-triazine with a purity of 95.46%.
[0029] All the products obtained above were added to 80.39g of toluene, and the temperature of the system was lowered to 5°C. Within 0.5h, 21.79g of thionyl chloride was added dropwise, and the temperature was controlled at 5-10°C. After the dropwise addition was completed, the temperature was raised to 63° C. and kept for 30 minutes. During the chlorination reaction process, acid gas will be released, and attention should be paid to ab...
Embodiment 3
[0032] Take 20.03g of melamine, add it to 100.15g of hydrochloric acid aqueous solution with a pH value of 4.5, start stirring, and raise the temperature to 100°C. During the reaction, 1M hydrochloric acid is continuously added to maintain the pH value, and the reaction is carried out for 7 hours. Then add 1M sodium hydroxide to adjust the reaction system to a pH value of about 7.0, then cool down to 5°C, filter, and dry to obtain 20.17g of solid, the main component of which is 4,6-diamino-2-hydroxy-1,3 ,5-triazine with a purity of 94.96%.
[0033] All the products obtained above were added to 80.37g of toluene, and the temperature of the system was lowered to 5°C. Within 0.5h, 21.54g of thionyl chloride was added dropwise, and the temperature was controlled at 5-10°C. After the dropwise addition was completed, the temperature was raised to 61° C. and kept for 30 minutes. During the chlorination reaction process, acid gas will be released, so the exhaust gas should be absorbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com