Cecropin F protein antibacterial peptide and application thereof
A technology of cecropin and antibacterial peptide, applied in the field of biomedicine, can solve problems such as the unsatisfactory treatment effect of antibacterial drugs, and achieve the effect of preventing and resisting bacterial infection, promoting wound healing, and reducing the use of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of embodiment 1 cecropin F protein antimicrobial peptide
[0035] S1. Weigh 1 times the molar equivalent of resin and put it into the reactor, add DCM (dichloromethane) to swell for half an hour, then remove the DCM, add 2 times the molar equivalent of the first amino acid in the sequence of SEQ ID NO.1, add 2 times Molar equivalent of DIEA, appropriate amount of DMF, DCM (appropriate amount means that the resin can be fully agitated), DIEA (diisopropylethylamine), DMF (dimethylformamide), DCM, nitrogen bubble reaction 60min. Then add about 5 times the molar equivalent of methanol, react for half an hour, remove the reaction solution, and wash with DMF and MEOH;
[0036] S2. Add the second amino acid in the sequence of SEQ ID NO.1 (also 2 times the molar equivalent) to the reactor, 2 times the molar equivalent of HBTU (1-hydroxy, benzo, trichlorazole tetramethyl hexafluorophosphate ) and DIEA, N2 bubbling reaction for half an hour, wash off the liquid, detec...
Embodiment 2
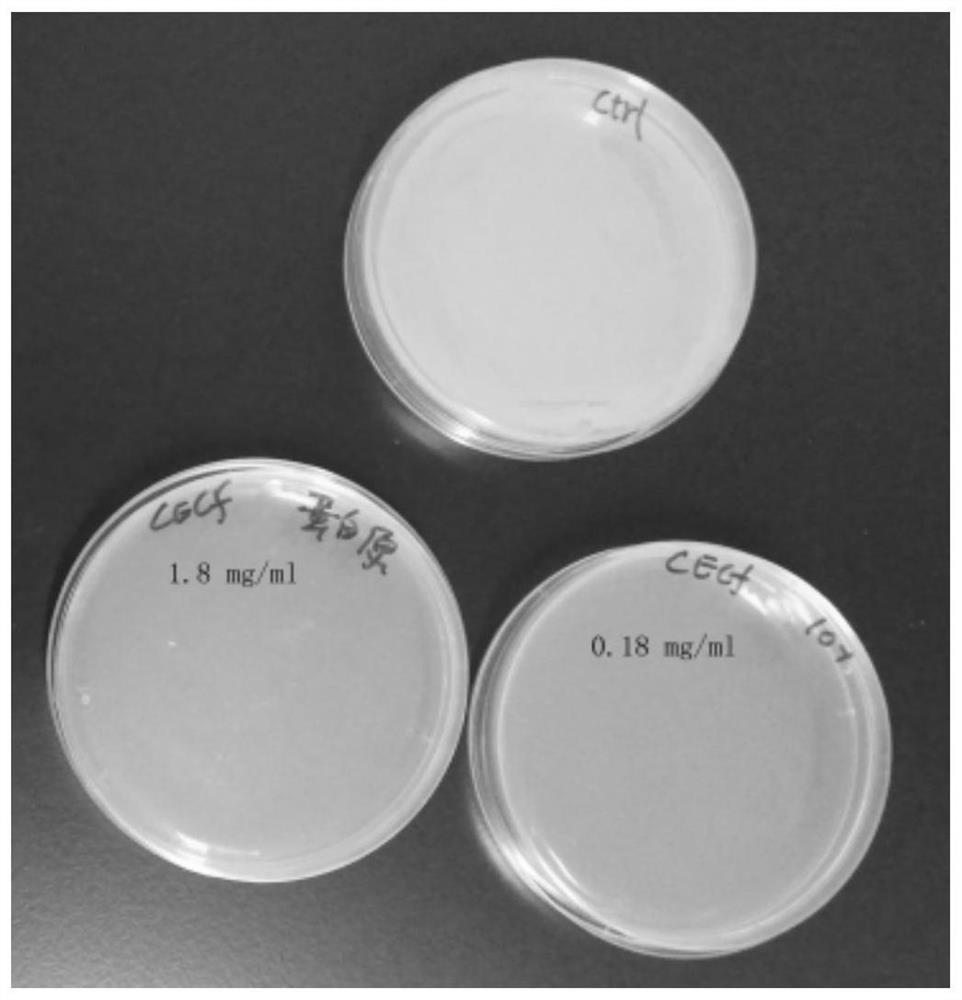
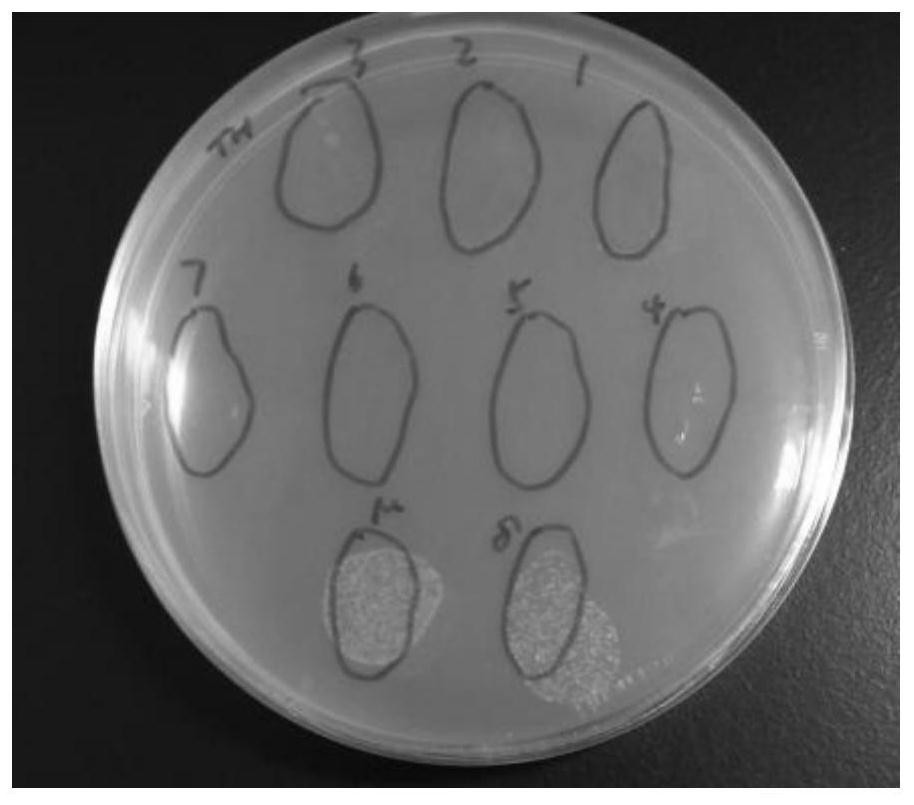
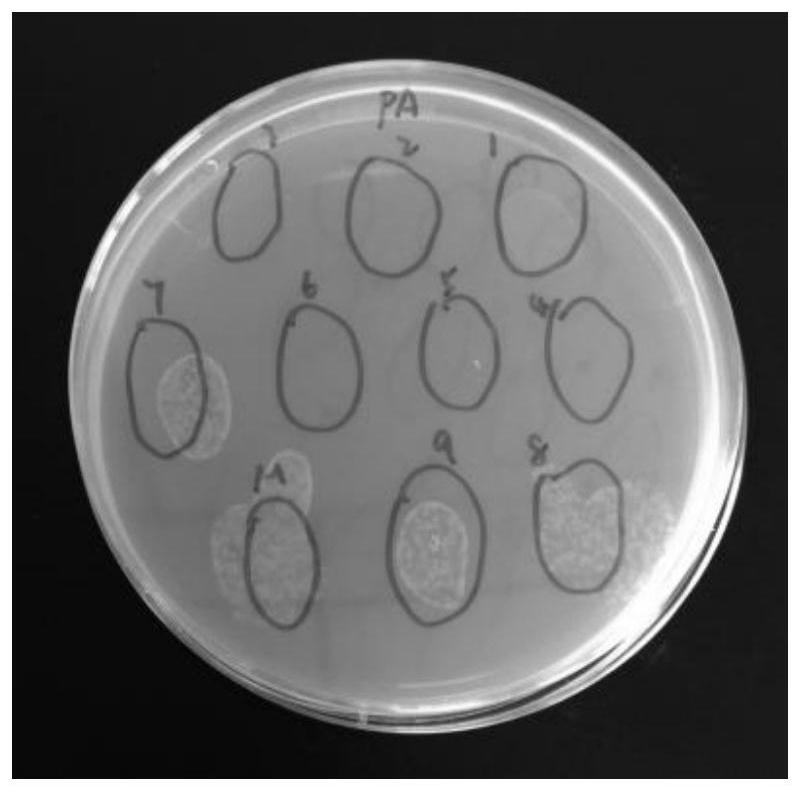
[0042] Example 2 Effects of CecF antimicrobial peptides on the propagation of Escherichia coli and Pseudomonas aeruginosa
[0043] 1. Preparation of CecF Antimicrobial Peptide Solution
[0044] Dissolve 0.5 mg of the CecF polypeptide synthesized in Example 1 in 500 ul sterile water to prepare 1.8 mg / ml and 0.18 mg / ml solutions, and place them in a -80 refrigerator for later use.
[0045] 2. Bacterial activation: Take 100ul of E. coli bacteria liquid and spread it on an LB (Luria-Bertani) plate without antibiotics, place it in a 37°C incubator, and incubate for 12 hours.
[0046]3. The application of CecF antimicrobial peptides to inhibit bacterial growth
[0047] (1) Take 100ul of the prepared CecF antimicrobial peptide solution, pick a single clone on the above-mentioned activated plate, put it in the antimicrobial peptide solution and mix it evenly, and then spread it on the LB plate without resistance. Incubate at 37°C for 12 hours, then remove for observation.
[0048] ...
Embodiment 3
[0054] Example 3 Effects of CecF Antimicrobial Peptides on Wound Healing in Mice
[0055] 1. Preparation of CecF Antimicrobial Peptide Solution
[0056] Dissolve 0.5 mg of CecF antimicrobial peptide synthesized in Example 1 in 2 ml of sterile water to make solutions of 400 ug / ml and 10 ug / ml, and place them in a -80 refrigerator for later use.
[0057] 2. The application of CecF antimicrobial peptide to promote wound healing
[0058] (1) Anesthesia: Kunming rats aged 6-8 weeks were selected, weighed, and injected intraperitoneally with chloral hydrate solution (10%) at 25 g / 100 ul.
[0059] (2) Shearing and depilation: After the mouse is anesthetized, fix it on the test bench, cut off the hair on the back and both sides of the mouse with scissors, apply the depilatory liquid evenly on the sheared area, wait for about 3-5 minutes, and use Gently wipe the skin with a cotton swab dampened with warm water to thoroughly remove the depilatory fluid and hair.
[0060] (3) Making w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com