Tetrahydro-beta-carboline dimer as well as preparation method and application thereof
A carboline dimer, carboline technology, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of incompletely clear pathogenesis, poor long-term efficacy, mental and economic burden, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
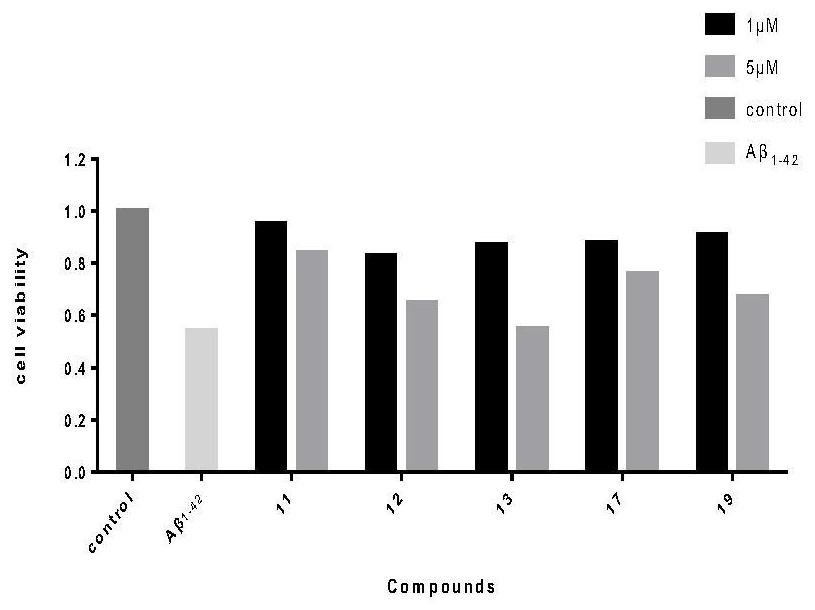
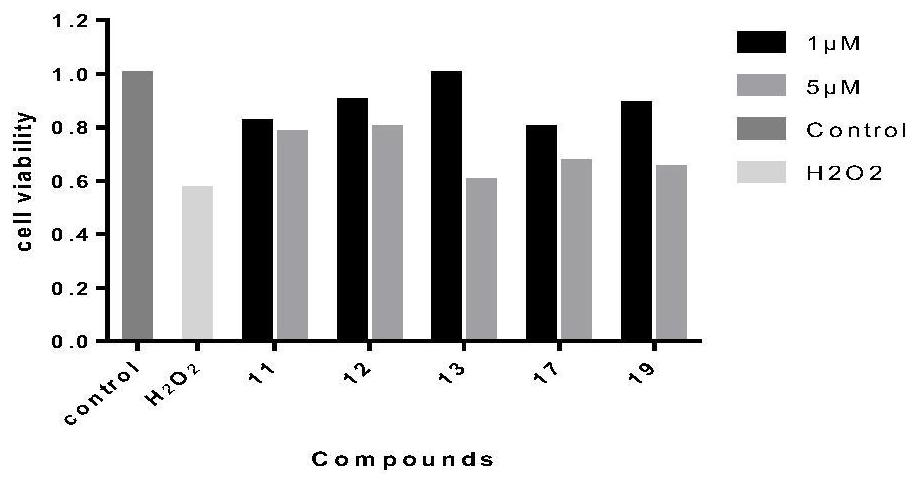
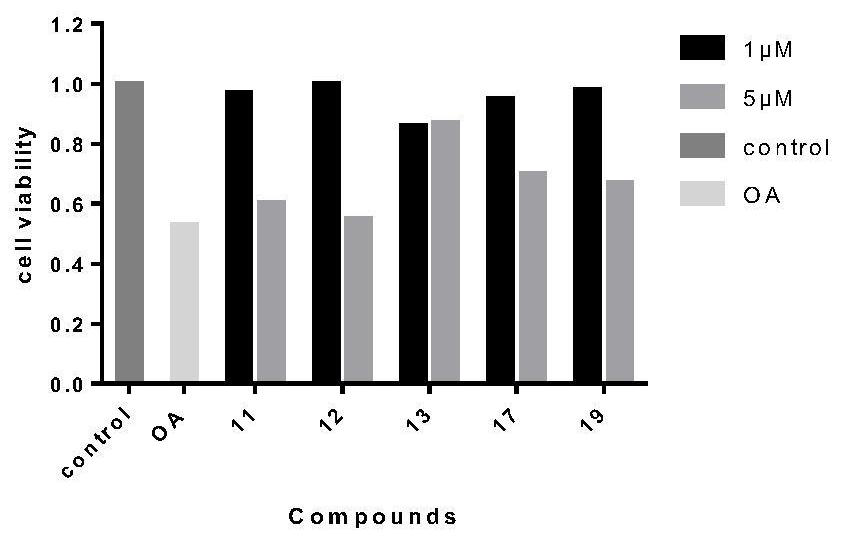
Image
Examples
Embodiment 1
[0068] 1-Methyl-3-methoxycarbonyl-1,2,3,4-tetrahydro-β-carbaline
[0069] a, L-tryptophan methyl ester
[0070]
[0071] Add SOCl dropwise to 450 mL methanol solution in ice bath 2 (4ml, 55.1mmol), after the dropwise addition was completed, the ice bath was removed, and after stirring at room temperature for 1h, L-tryptophan (5g, 24.5mmol) was added, stirred at room temperature overnight, TLC detected that the reaction was complete, and the solvent was evaporated under reduced pressure to obtain Add a small amount of water to dissolve the white solid, repeat several times, then add a certain amount of water, slowly add ammonia water to adjust the pH=7-8, add dichloromethane to dissolve, extract 3 times, 30ml each time, combine the organic layer, After drying with anhydrous sodium sulfate and concentrating dichloromethane, 3.7 g of viscous solid was obtained, with a yield of 69.2%. ESI-MS m / z:219.1[M+H] + , The relative molecular mass is 218.
[0072] b, Preparation of 1...
Embodiment 2
[0076] Preparation of 1-phenyl-3-methoxycarbonyl-1,2,3,4-tetrahydro-β-carbaline (compound 31)
[0077]
[0078] L-tryptophan methyl ester (1.0g, 4.58mmol) was dissolved in 20ml of anhydrous DCM, and trifluoroacetic acid TFA (2ml, 26.8mmol) was added dropwise under ice-bath conditions. After the dropwise addition, the ice bath was removed. After stirring at room temperature for 0.5 h, benzaldehyde (2 ml, 19.6 mmol) was added and reacted at room temperature for 12 h. TLC detected the completion of the reaction, adjusted the pH to 8-9 with ammonia water, extracted the reaction solution with 40 mL of dichloromethane, washed 3 times with saturated brine, 20 mL each time, dried the organic layer with anhydrous sodium sulfate, and evaporated the solvent under reduced pressure. Separation and purification by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate (1:2), to obtain 0.68 g of a light brown solid with a yield of 48.4%. Identified as 1-phenyl-3-...
Embodiment 3
[0080] Preparation of 2-chloro-1-(1-methyl-1,2,3,4-tetrahydro-β-carbaline-3-carboxylic acid methyl ester)ethanone (compound 37)
[0081]
[0082] Dissolve compound 1 (200mg, 0.820mmol) in anhydrous CHCl 3 , add NaHCO 3 (82mg, 0.982mmol / L), after stirring for 30min, slowly added 2-chloroacetyl chloride (97μL, 1.23mmol) dissolved by an appropriate amount of chloroform, and reacted at room temperature for 6h under nitrogen dry conditions, after the reaction was detected by TLC, The reaction solution was filtered, the solvent was evaporated under reduced pressure, an appropriate amount of dichloromethane was added, and saturated NaHCO 3 Solution extraction, multiple extractions, combined organic layers, distilled off the solvent under reduced pressure to obtain a brown viscous substance, which was separated and purified by silica gel column chromatography, the eluent was dichloromethane:methanol (50:1), and 160 mg of light yellow powder was obtained. The yield was 60.9%. Ide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com