Human peripheral blood immune cell cryopreservation efficient stabilizer, application and cryopreservation method
An immune cell and stabilizer technology, which is applied in the field of high-efficiency stabilizer for cryopreservation of human peripheral blood immune cells and its application and cryopreservation, can solve problems such as allergic reactions, increase the risk of pathogenic contamination, and the impact of immunotherapy, so as to reduce the possibility of, Avoid adverse reactions and toxic side effects and ensure the effect of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]This example is used to introduce a high-efficiency stabilizer for cryopreservation of human peripheral blood immune cells and its application and cryopreservation method of the present invention. The cryopreservation agent of the present invention is a serum-free cell cryopreservation solution that does not contain animal serum, dimethyl sulfoxide (DMSO) and animal source components, does not introduce exogenous animal source proteins, reduces the possibility of animal source contamination, and also It avoids adverse reactions and toxic side effects when the cells are reinfused back to the patient after resuscitation, and will not cause harm to human cell immunotherapy, and is safe and effective.
[0041] A high-efficiency stabilizer for cryopreservation of human peripheral blood immune cells, the main components of which are proline, sucrose, polyvinylpyrrolidone, ethylene glycol, ectoine, polyvinyl alcohol and basal cell culture fluid without phenol red; The proline c...
Embodiment example 1
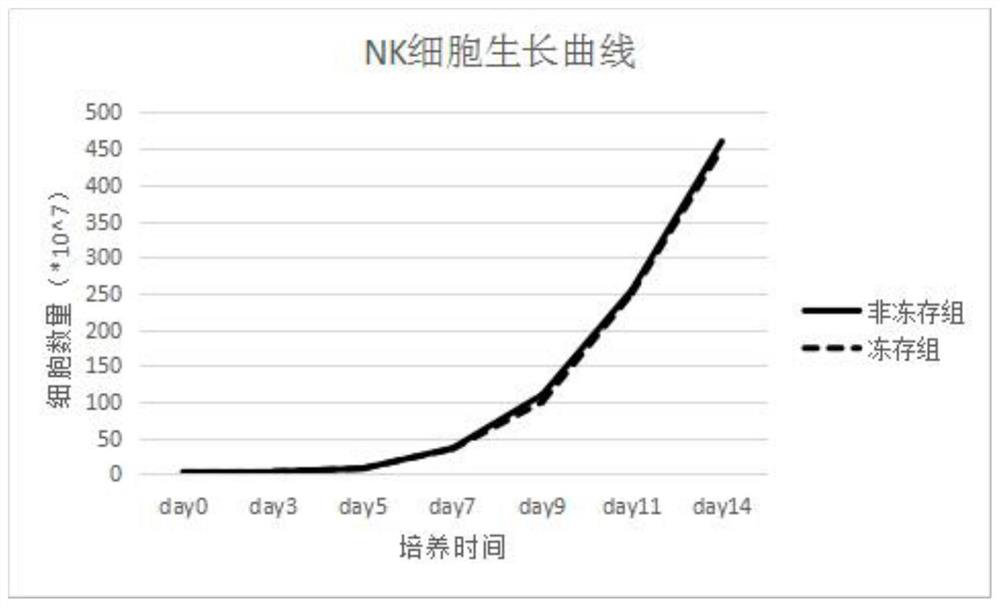
[0074] Implementation case 1 result:
[0075]
[0076]
[0077] As another realization, a high-efficiency stabilizer for cryopreservation of human peripheral blood immune cells, in which the concentration of proline is 1.5% (W / V), and the concentration of sucrose is 5.5% (W / V), the concentration of polyvinylpyrrolidone is 3% (W / V), the ratio of ethylene glycol is 6% (V / V), and the concentration of ectoine is 5% (W / V), the polyvinyl alcohol concentration is 2% (W / V), and the ratio of the basic cell culture solution is 94% (V / V). The preparation method is the same as before.
[0078] As another realization, a high-efficiency stabilizer for cryopreservation of human peripheral blood immune cells, in the cryopreservation agent, the concentration of proline is 2% (W / V), and the concentration of sucrose is 6%. (W / V), the concentration of the polyvinylpyrrolidone is 6% (W / V), the ratio of the ethylene glycol is 8% (V / V), the ectoine concentration is 3% (W / V), the polyvinyl ...
Embodiment 2
[0087] (1) Separation of human peripheral blood immune cells
[0088] Collect 40ml of human peripheral blood anticoagulated with heparin sodium solution from a healthy volunteer, slowly pour it into two centrifuge tubes containing 15ml of lymphocyte separation medium, and centrifuge at room temperature with a centrifugal force of 800xg for 15 minutes. After centrifugation, the blood is divided into 4 layers. Collect the upper layer of plasma with a sterile pipette, pour it into a sterilized centrifuge tube, heat the plasma at 56°C for 30 minutes, store it in the refrigerator for later use, absorb the buffy coat cells, and wash with PBS for 2 Second-rate.
[0089] (2) Preparation of cryopreservation solution for the control group
[0090] Traditional technology serum group: use 10% fetal bovine serum + 10% DMSO + 80% RPMI1640 culture solution.
[0091] Commercially available commercial DMSO group: cell cryopreservation medium LABOBANKER2 (NO: BLB-2, without serum).
[0092] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com