3-carbonyl hyperin, extraction method and application thereof in preparation of antitumor drugs
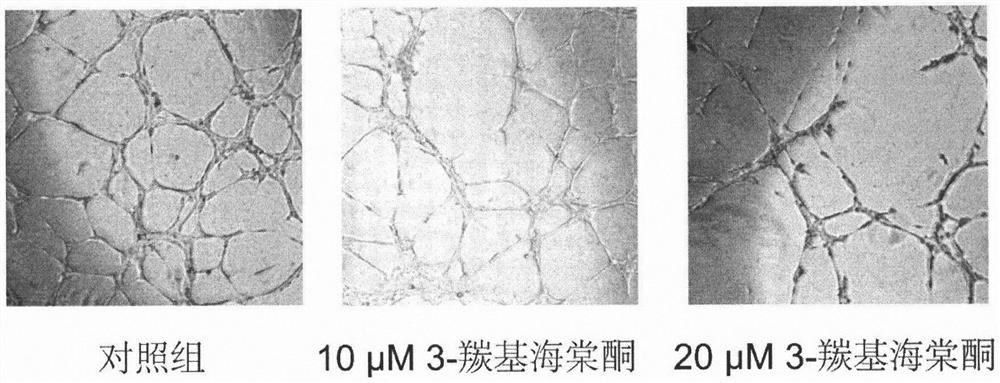
A carbonyl begonia ketone and anti-tumor drug technology, applied in the application field of preparing drugs, can solve the problems of unreported pharmacological activity, etc., and achieve the effect of inhibiting the ability of clone formation, inhibiting tumor angiogenesis, and inhibiting tumor growth and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Extraction and identification of 3-carbonyl begonia ketone
[0022] Take 10 kg of Kunming mountain crabapple rhizomes, crush them, pass through a 10-mesh sieve, and ultrasonically extract them three times with 500 L of 95% ethanol, with a starting power of 3 kw and a frequency of 40 kHz, filter and collect the extract, and concentrate the extract under reduced pressure until it has no alcohol smell to obtain a concentrate. The concentrated solution was sequentially extracted with petroleum ether and dichloromethane, the dichloromethane extract was collected, washed 3 times with 2 times the amount of saturated sodium bicarbonate aqueous solution, and the dichloromethane extract was concentrated to a small volume to obtain a sample concentrate. Then mix dichloromethane-methanol-water according to 6:4:1, let stand for 24h to separate layers, take the upper phase as the stationary phase and pump it into the high-speed countercurrent chromatograph, and the lower phase as the ...
Embodiment 2
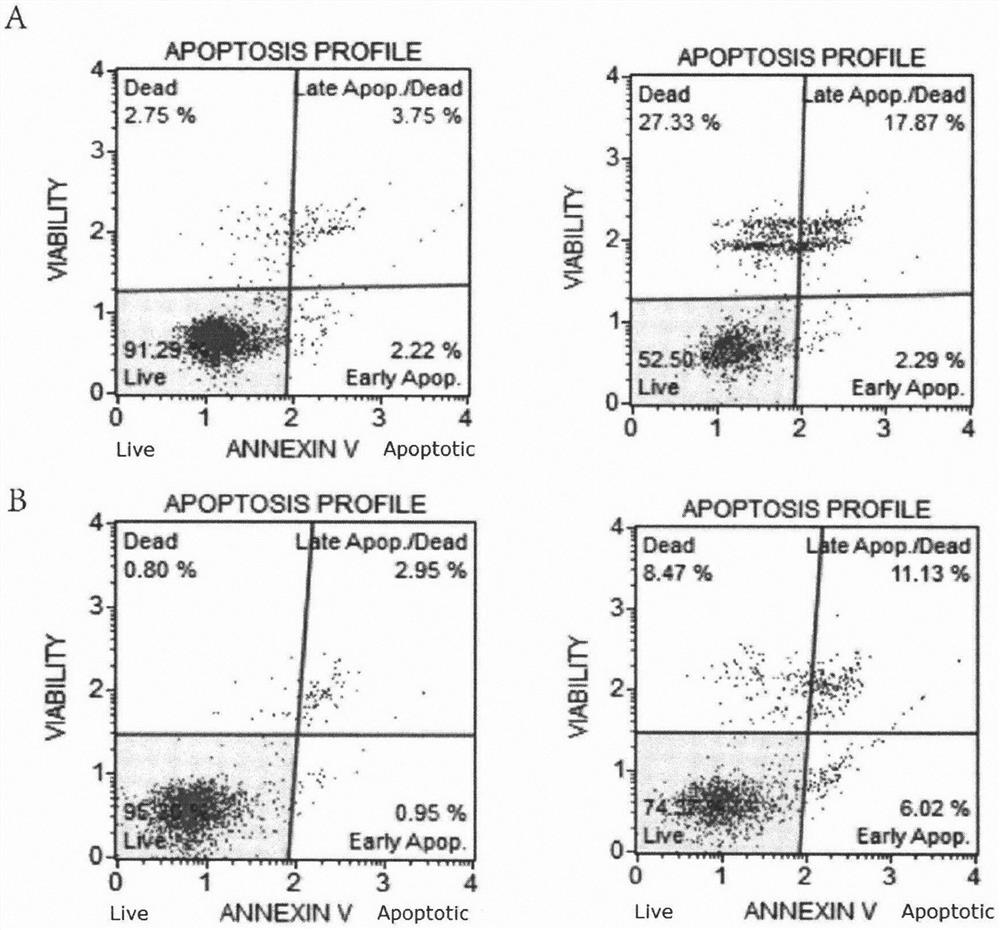
[0030] Detecting the antitumor activity of 3-carbonyl begonia ketone and begonia ketone in vitro by MTT assay
[0031] Experimental principle: MTT, chemical name 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), is a general cell staining agent. There is a succinate dehydrogenase related to NADP in the mitochondria of living cells, which can reduce exogenous yellow MTT to insoluble blue-purple crystal formazan (Formazane), which is deposited in the cells. In dead cells, this enzyme disappears, and MTT are not restored. After dissolving Formazane with DMSO, use a microplate reader to detect the change in optical density at a wavelength of 550nm, which can display the survival and growth of cells in the detection system. People often use MTT method to measure the growth inhibitory effect of test drugs on cells, and then evaluate cytotoxicity.
[0032] The purpose of the experiment: To detect the inhibitory activity of 3-carbonyl begonia ketone and the existing com...
Embodiment 3
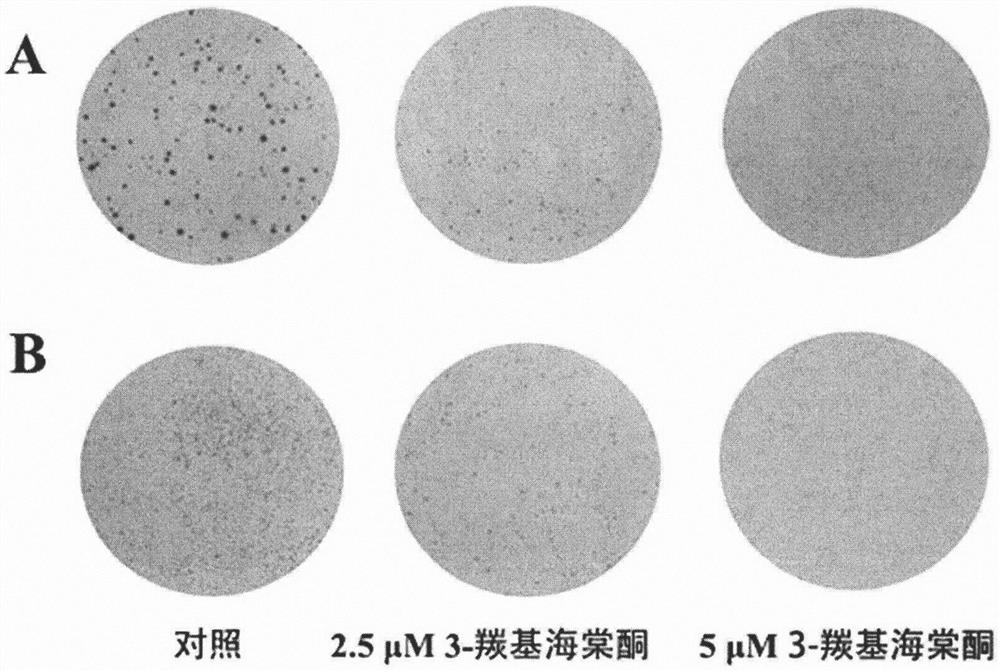
[0040] Effects of 3-carbonyl begonia ketone on the colony formation ability of lung cancer A549 and H1299 cells
[0041] Experimental principle: Plate colony formation assay is one of the effective methods to measure cell proliferation ability, and the cells that form clones are adherent cells with strong proliferative activity. The cell clone formation rate indicates the number of adherent cells that survive and form clones after inoculation of cells, which can reflect two important traits of cell population dependence and proliferation ability. The clonogenic ability of the tumor cells treated with the compound shows the anti-tumor effect of the compound.
[0042] Experimental procedure: Lung cancer A549 and H1299 cells were inoculated in a six-well plate at 500 cells / well, respectively, and blank control, 2.5 μM 3-carbonyl begonia ketone and 5 μM 3-carbonyl begonia ketone were added. Culture each well in 2mL conventional medium, change the medium every 2-4 days, stop the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com