Fusion protein containing fluorescent protein fragment and application of fusion protein
A fusion protein and fluorescent protein technology, applied in the field of fusion proteins, can solve problems such as difficult separation, strong hydrophobicity, and low specific gravity of the target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
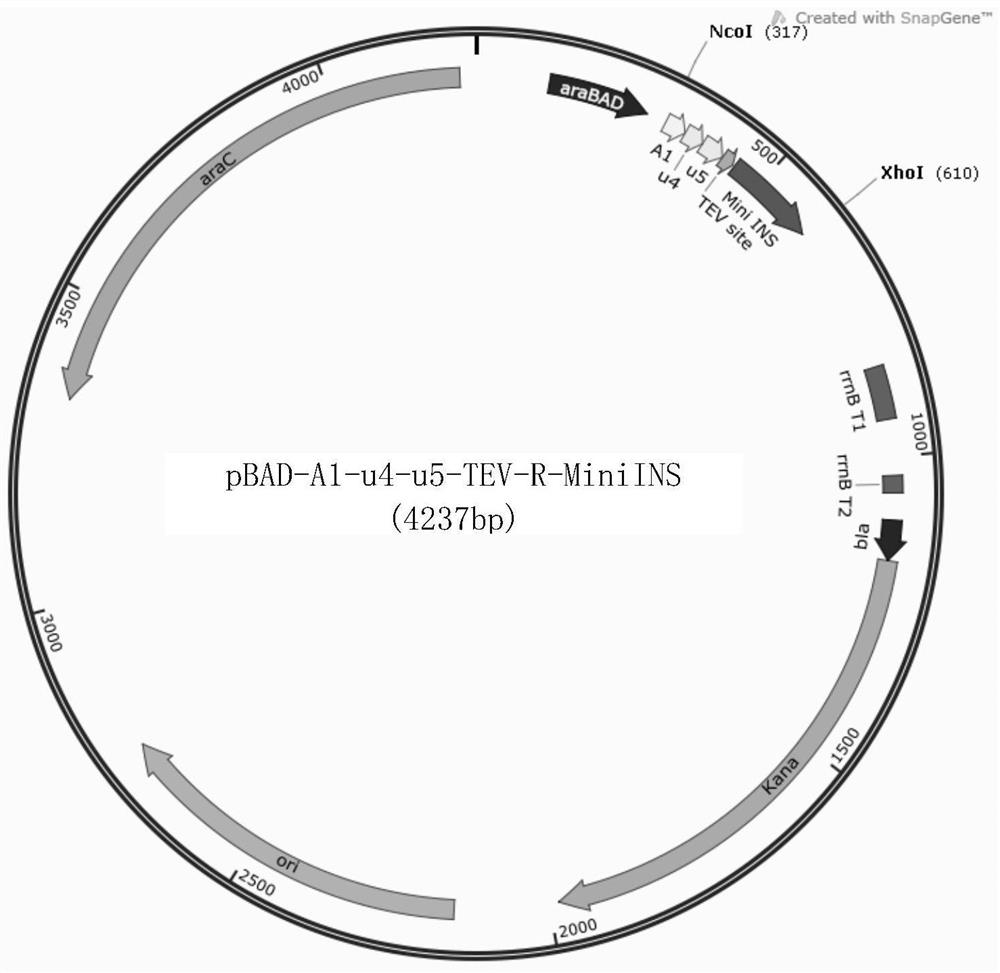
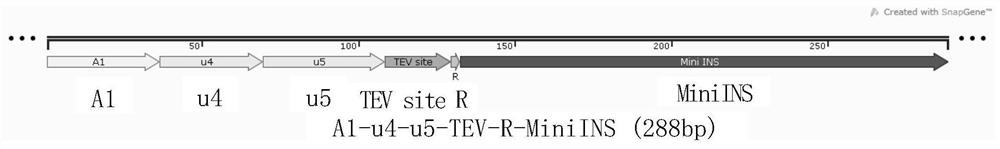
[0217] Example 1 Construction of expression vector A1-u4-u5-TEV-R-MiniINS
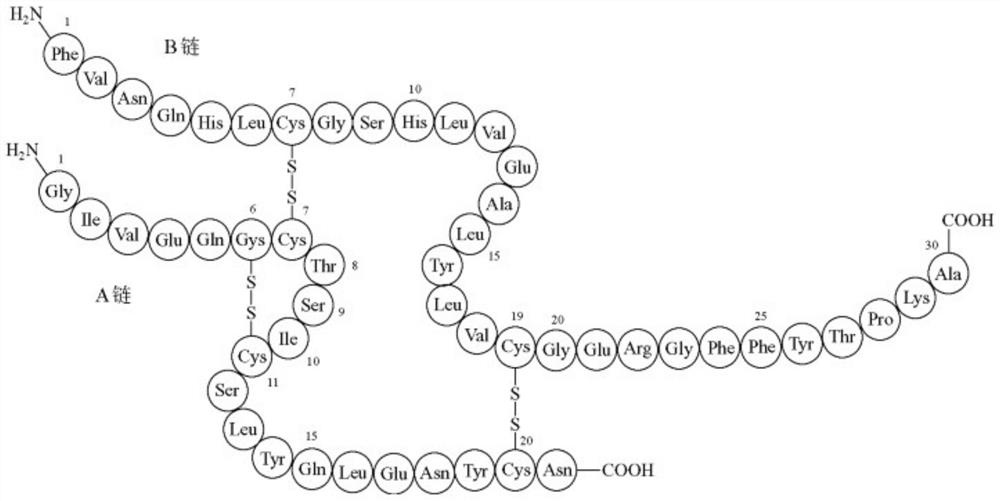
[0218] The expression construct A1-u4-u5-TEV-R-MiniINS contains the gene encoding human insulin protein fused to the C-terminus of A1-u4-u5. The connecting peptide between A1-u4-u5 and the insulin protein MiniINS is the octapeptide Glu-Asn-Leu-Tyr-Phe-Gln-Gly-Arg. The octapeptide can be hydrolyzed by trypsin at the carboxyl end of Arg, and can also be hydrolyzed by TEV protease between Gln-Gly. The DNA sequence of the octapeptide is codon-optimized, which can realize high-level expression of functional proteins in Escherichia coli.
[0219] The fusion recombinant protein fragments used were all synthesized by GenScript and loaded into the pUC57 vector, and "A1-u4-u5-TEV-R-MiniINS" was synthesized from the synthetic vector "pUC57-A1-u4" using restriction enzymes NcoI and XhoI -u5-TEV-R-MiniINS”, cut the expression vector “pBAD / His A(KanaR)” with restriction endonucleases NcoI and XhoI at the same time...
Embodiment 2
[0220] Example 2 Construction of expression construct A1-u4-u5-TEV-R-GLP1
[0221] Construct and synthesize pUC57-A1-u4-u5-TEV-R-GLP1 as in Example 1, and cut the expression vector "pBAD / His A(KanaR)" with restriction enzymes NcoI and XhoI at the same time, and digest the product Separation by agarose electrophoresis, extraction using an agarose gel DNA recovery kit, and finally linking the two DNA fragments using T4 DNA ligase. The ligated product was chemically transformed into Escherichia coli Top10 cells, and the transformed cells were cultured on LB agar medium containing 50 μg / mL kanamycin (10 g / L yeast peptone, 5 g / L yeast extract powder, 10g / L NaCl, 1.5% agar) overnight. Pick 3 viable colonies and culture overnight in 5 mL liquid LB medium (10 g / L yeast peptone, 5 g / L yeast extract powder, 10 g / L NaCl) containing 50 μg / mL kanamycin, and use plasmid mini-extraction Kit for plasmid extraction. Then, the extracted plasmid was sequenced using the sequencing oligonucleot...
Embodiment 3
[0222] Example 3 Expression, isolation and purification of A1-u4-u5-TEV-R-MiniINS fusion protein
[0223]In order to express the fusion fragment of A1-u4-u5-TEV-R-MiniINS containing the amino acid sequence of SEQ ID NO.:21. Plasmid pBAD-A1-u4-u5-TEV-R-MiniINS and pyrrolysine aminoacyl tRNA synthetase plasmid pEvol-pylRs-pylT (wherein, pyrrolysine aminoacyl tRNA synthetase plasmid pEvol- pylRs-pylT is used to express aminoacyl tRNA synthetase and tRNA, as shown in Example 1 of Patent Application No. 2011103886241) and transformed into E. coli strain Top10 together. The transformation solution was placed on LB agar medium containing 25 μg / mL kanamycin and 17 μg / mL chloramphenicol overnight. Pick a single colony and culture overnight in LB liquid medium containing 25 μg / mL kanamycin and 17 μg / mL chloramphenicol. Then, the overnight culture was inoculated into 100 mL of TB medium containing 25 μg / mL of kanamycin and 17 μg / mL of chloramphenicol (liquid TB medium: 12 g / L yeast pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com