A kind of pramipexole sustained-release microsphere and preparation method thereof
A technology of slow-release microspheres and pramipexole, which is applied in the field of medicine, can solve the problems of cumbersome process, reduce solubility in water, and affect the particle size of microspheres, and achieve the advantages of simple preparation process, improved stability, and high encapsulation efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of pramipexole sustained-release preparation, comprising the following steps:
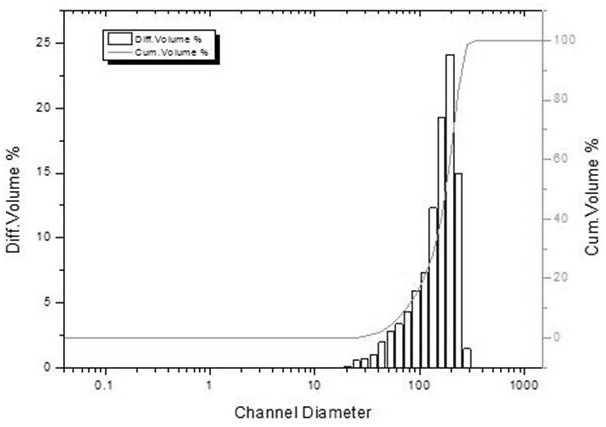
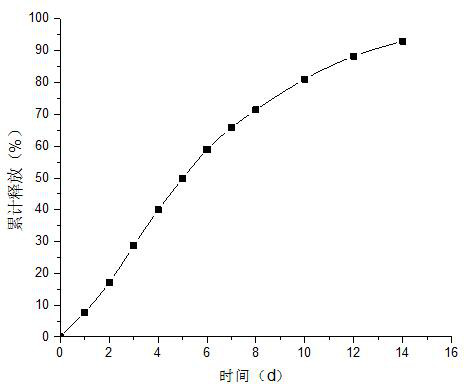
[0042]Weigh 1.00g pramipexole dihydroxynaptate and 1.00g PLGA (LA:GA=50:50), add 6ml dichloromethane and stir to dissolve as oil phase; prepare 0.8% PVA aqueous solution as water phase, cool to 10-25°C for later use; slowly add the oil phase to 0.8% PVA aqueous solution, and emulsify at 1300rpm / min for 1min under the action of a shearing machine; after emulsification, solidify the emulsified microspheres at room temperature, Stir for 4h to evaporate the solvent; transfer the microspheres to an ethanol-water (20 / 80) solution containing 0.25% poloxamer and continue stirring for 1h, sieve the obtained microsphere suspension through a filter, and wash the microspheres with water. After 3 times of spheres, the pramipexole sustained-release microspheres were obtained by vacuum drying.
Embodiment 2
[0044] A preparation method of pramipexole sustained-release preparation, comprising the following steps:
[0045] Weigh 0.60g pramipexole pashydronaphate and 1.25g PLGA (LA:GA=50:50), add 7ml dichloromethane and stir to dissolve as oil phase; prepare 0.8% PVA aqueous solution as water phase, cool to 10-25°C for later use; slowly add the oil phase to 0.8% PVA aqueous solution, and emulsify at 1300rpm / min for 1min under the action of a shearing machine; after emulsification, solidify the emulsified microspheres at room temperature, Stir for 4h to evaporate the solvent; transfer the microspheres to an ethanol-water (20 / 80) solution containing 0.25% poloxamer and continue stirring for 1h, sieve the obtained microsphere suspension through a filter, and wash the microspheres with water. After 3 times of spheres, the pramipexole sustained-release microspheres were obtained by vacuum drying.
Embodiment 3
[0047] A preparation method of pramipexole sustained-release preparation, comprising the following steps:
[0048] Weigh 0.30g pramipexole pashydronaphate and 0.70g PLGA (LA:GA=50:50), add 3ml dichloromethane and stir to dissolve as oil phase; prepare 0.8% PVA aqueous solution as water phase, cool to 10-25°C for later use; slowly add the oil phase to 0.8% PVA aqueous solution, and emulsify at 1300rpm / min for 1min under the action of a shearing machine; after emulsification, solidify the emulsified microspheres at room temperature, Stir for 4h to evaporate the solvent; transfer the microspheres to an ethanol-water (20 / 80) solution containing 0.25% poloxamer and continue stirring for 1h, sieve the obtained microsphere suspension through a filter, and wash the microspheres with water. After 3 times of spheres, the pramipexole sustained-release microspheres were obtained by vacuum drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com