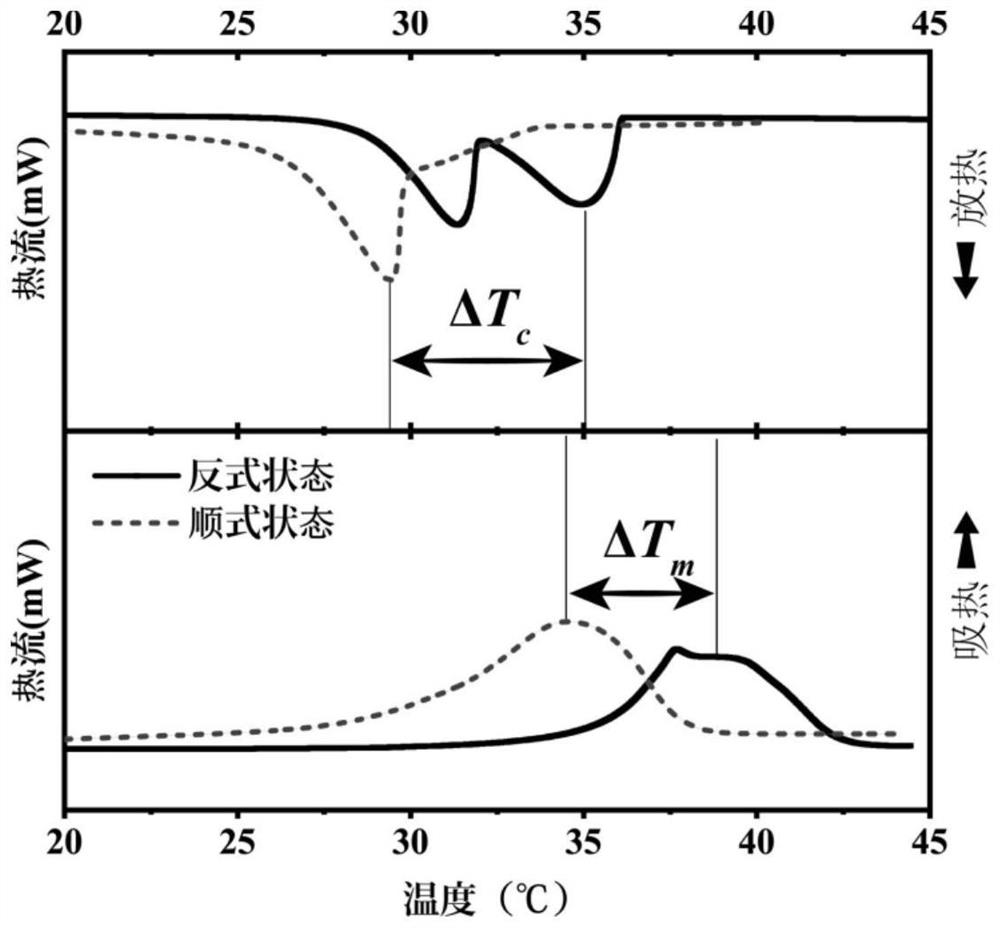
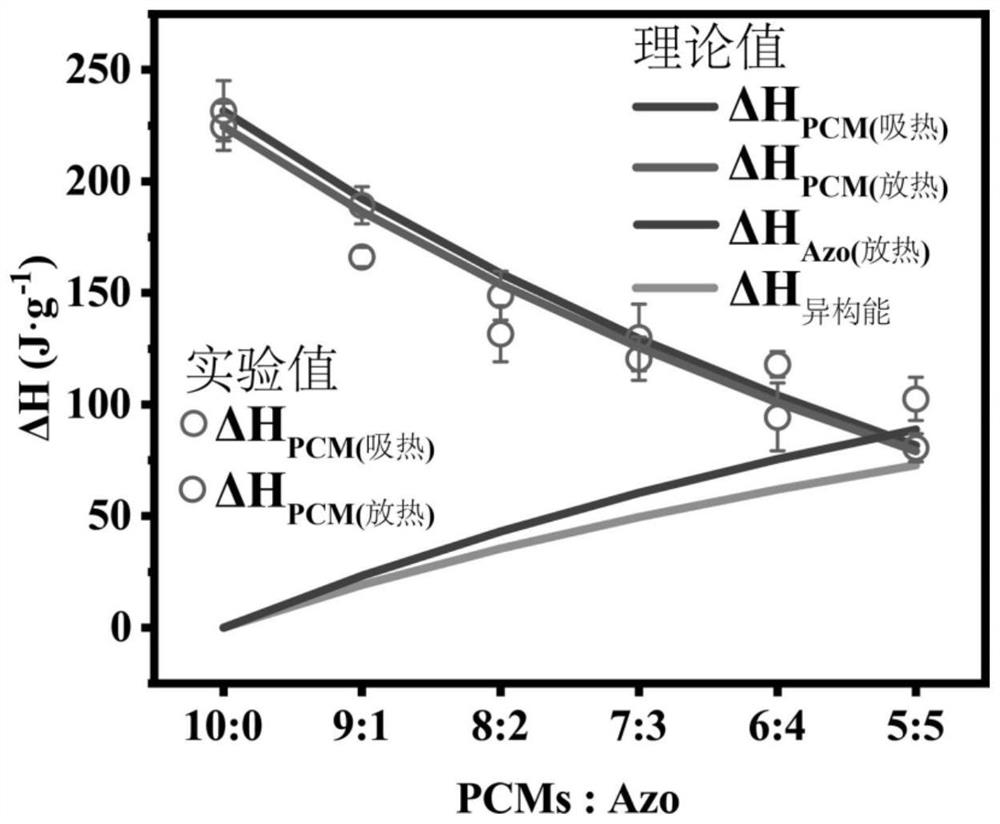
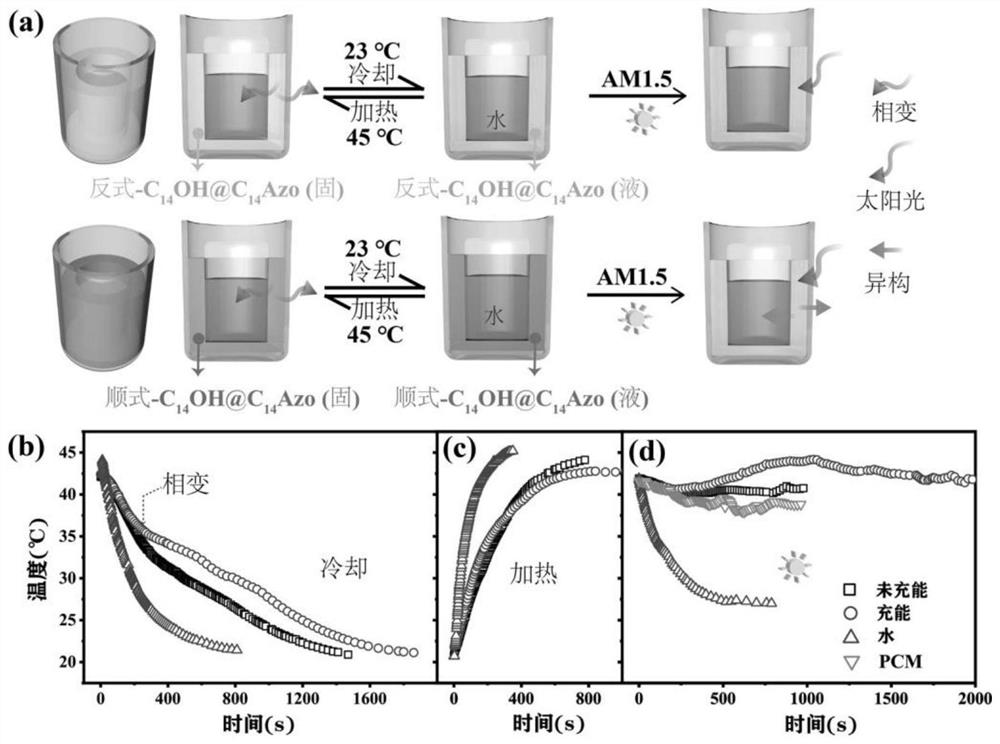
Preparation method and application of azobenzene-based light energy storage phase change material
An alkoxyazobenzene storage and energy storage phase change technology, which is applied in the direction of materials, chemical instruments and methods for analysis and heat exchange through chemical reaction of materials, so as to increase the energy storage effect and improve the degree of isomerization. , the effect of accelerating the speed of heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 4-butoxyazobenzene
[0031] Dissolve p-hydroxyazobenzene and 1-bromo-n-butane, potassium hydroxide and potassium iodide with N,N-dimethylformamide (DMF) and pour them into a three-necked flask at a molar ratio of 1:2:3: 1 was added, stirred and heated to 80°C, and reacted for 12h. The resulting solution was distilled off under reduced pressure to remove the solvent DMF, then extracted with dichloromethane, the organic layer was taken, evaporated under reduced pressure to remove dichloromethane. Finally, a eluent of petroleum ether: ethyl acetate = 7:1 (v / v) was used Pass through the column, take the middle layer, and remove the eluent by rotary evaporation to obtain the product 4-butoxyazobenzene.
[0032] Blend the 4-butoxyazobenzene obtained in the first step with paraffin wax at a molar ratio of 2:8, heat and stir at 70°C for 2 hours, and completely mix the two substances evenly.
Embodiment 2
[0034] Synthesis of 4-octyloxyazobenzene
[0035] Dissolve p-hydroxyazobenzene, 1-bromo-n-octane, anhydrous cesium carbonate and potassium iodide in N,N-dimethylformamide (DMF) and pour them into a three-necked flask, with a molar ratio of 1:2:3 :1 was added, stirred and heated to 80°C, and reacted for 8h. The resulting solution was distilled off under reduced pressure to remove the solvent DMF, then extracted with dichloromethane, the organic layer was taken, evaporated under reduced pressure to remove dichloromethane. Finally, the eluent of petroleum ether: ethyl acetate = 6:1 (v / v) was used Pass through the column, take the middle layer, and remove the eluent by rotary evaporation to obtain the product 4-octyloxyazobenzene.
[0036] The 4-octyloxyazobenzene and octadecanoic acid obtained in the first step were blended at a molar ratio of 5:5, heated and stirred at 80°C for 2 hours, and the two substances were completely mixed evenly.
Embodiment 3
[0038] Synthesis of 4-tetradecyloxyazobenzene
[0039] Dissolve p-hydroxyazobenzene and 1-bromo-n-tetradecane, anhydrous sodium carbonate and potassium iodide with N,N-dimethylformamide (DMF) and pour them into a three-necked flask at a molar ratio of 1:2.5: Add 3:2, stir and heat to 80°C, and react for 12h. The resulting solution was distilled off under reduced pressure to remove the solvent DMF, then extracted with dichloromethane, the organic layer was taken, evaporated under reduced pressure to remove dichloromethane. Finally, the eluent of petroleum ether: ethyl acetate = 6:1 (v / v) was used Pass through the column, take the middle layer, and remove the eluent by rotary evaporation to obtain the product 4-tetradecyloxyazobenzene.
[0040] Blend the 4-tetradecyloxyazobenzene obtained in the first step with tetradecyl alcohol in a molar ratio of 3:7, heat and stir at 80°C for 2 hours, and the two substances are completely mixed evenly.
[0041] Example 3
[0042] Synthesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com