Bufalin (BUF)-carrying nano liposome modified by hyaluronic acid as well as preparation method and application of BUF-carrying nano liposome
A technology of hyaluronic acid modification and nano-liposome, which is applied in the field of medicine to achieve the effects of prolonging the efficacy, increasing the accumulation and increasing the sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 HA-DOPE
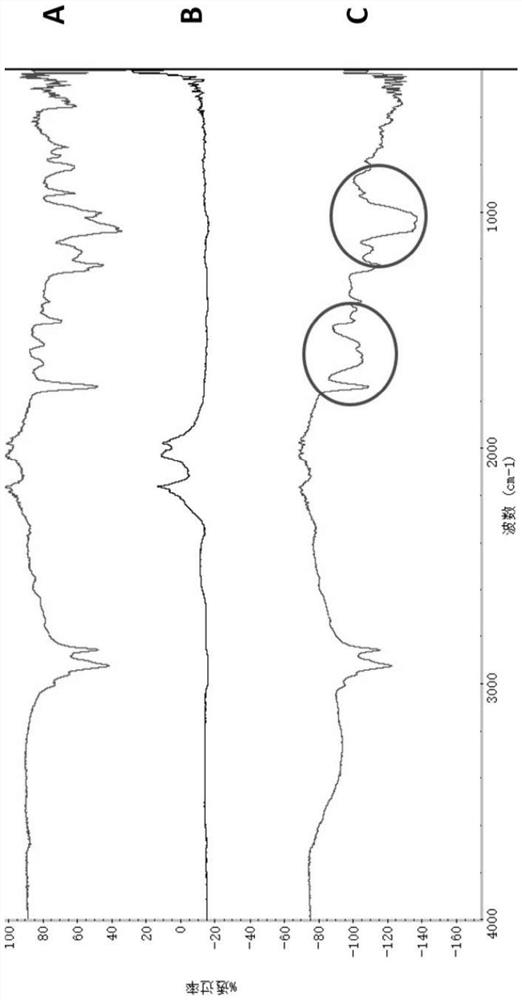
[0037] Hyaluronic acid (HA) activation solution was prepared by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS), containing phosphatidyl The suspension of ethanolamine (DOPE) is added to the activation solution to activate the reaction, and the unreacted DOPE, EDC and other reagents are removed by dialysis to prepare HA-DOPE. The HA-DOPE freeze-dried powder and KCl powder were mixed and ground at a ratio of 1:100, sieved to make the particle size below 2.5 μm, and put into a tablet former. Pressurized (5-10t / cm 2 ) in about 3 minutes to obtain a transparent sheet, which can be tested on the machine. The infrared spectra of HA, DOPE and HA-DOPE are shown in the figure, in A 1690~1650cm -1 The multiplet is the stretching vibration peak of C=O bond and amide in HA, 2910cm in B -1 , 1780cm -1 , 1380.97cm -1 It is the characteristic absorption peak of long-chain methylene in DOPE, 1735.44 cm ...
Embodiment 2
[0038] Example 2 Preparation of HA / lip-BUF
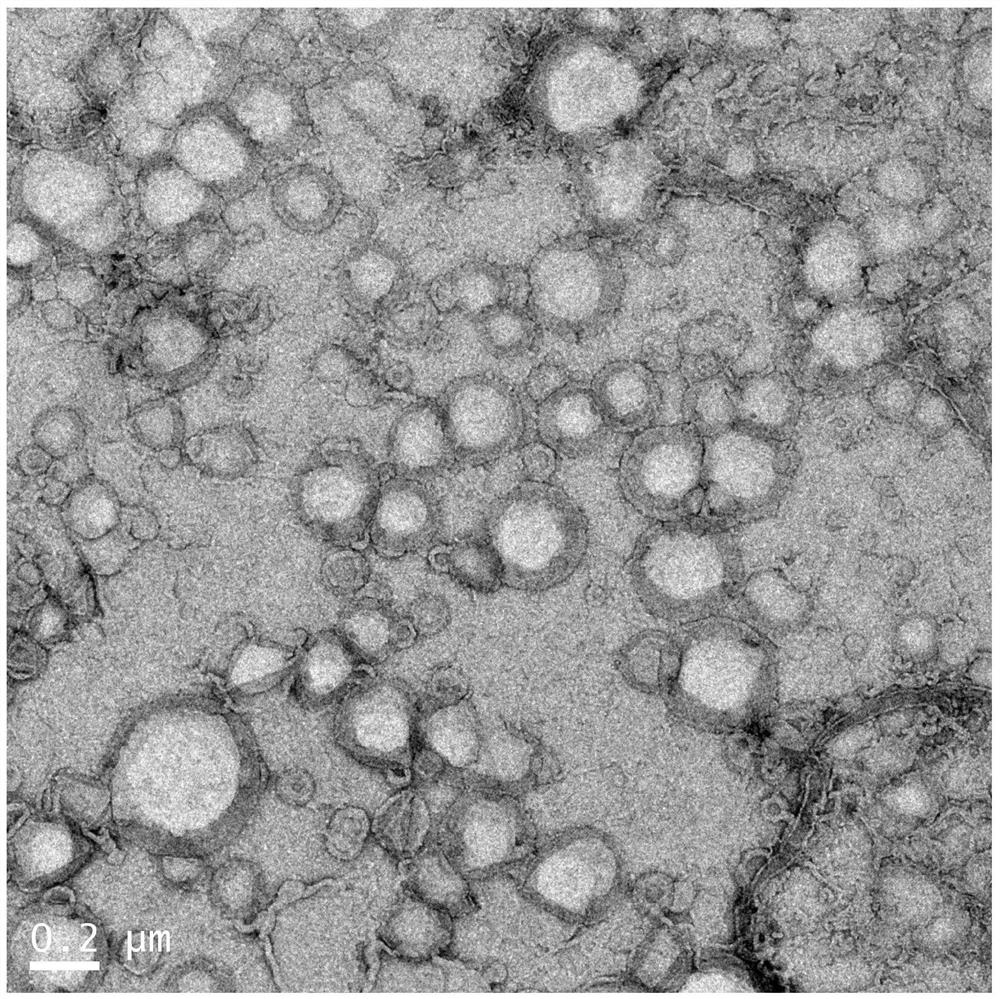
[0039] Precisely weigh 72 mg of S100 and 24 mg of cholesterol in a round bottom flask, add 10 mL of chloroform and a certain amount of BUF (and HA-DOPE), stir for 10 min to form a uniform solution, remove the chloroform by rotary evaporation in a water bath at 30-60 ° C, and form a uniform transparent film. Then add 9 mL of chloroform and 3 mL of PBS, shake vigorously to mix, and sonicate for 5 min until a stable W / O phase is formed. Rotary steam in a water bath at 30-60°C until it becomes flocculent, then add 5 mL of PBS and continue rotating until the hydration is complete (hydration for 1 hour), and the bufalin liposome is obtained. Probe ultrasound for 10min, 10-30%W, 5-10s on, 5s off, standby. After the prepared liposomes were diluted to an appropriate concentration with distilled water, the particle diameter and PDI of the liposomes were measured with a Malvern Zetasizer particle size analyzer. The morphology and size of the...
Embodiment 3
[0040] Embodiment 3 measures the encapsulation efficiency of loading BUF liposome
[0041] Column: C18 VanGuard Pre-column, 1.7μm, 2.1mm X 5mm; mobile phase - acetonitrile: water = 4: 6; flow rate: 0.5mL / min; column temperature: 10-40°C; detection wavelength: 280nm; injection volume: 2μL. The encapsulation efficiency of BUF-loaded liposomes was determined by ultrafiltration centrifugation.
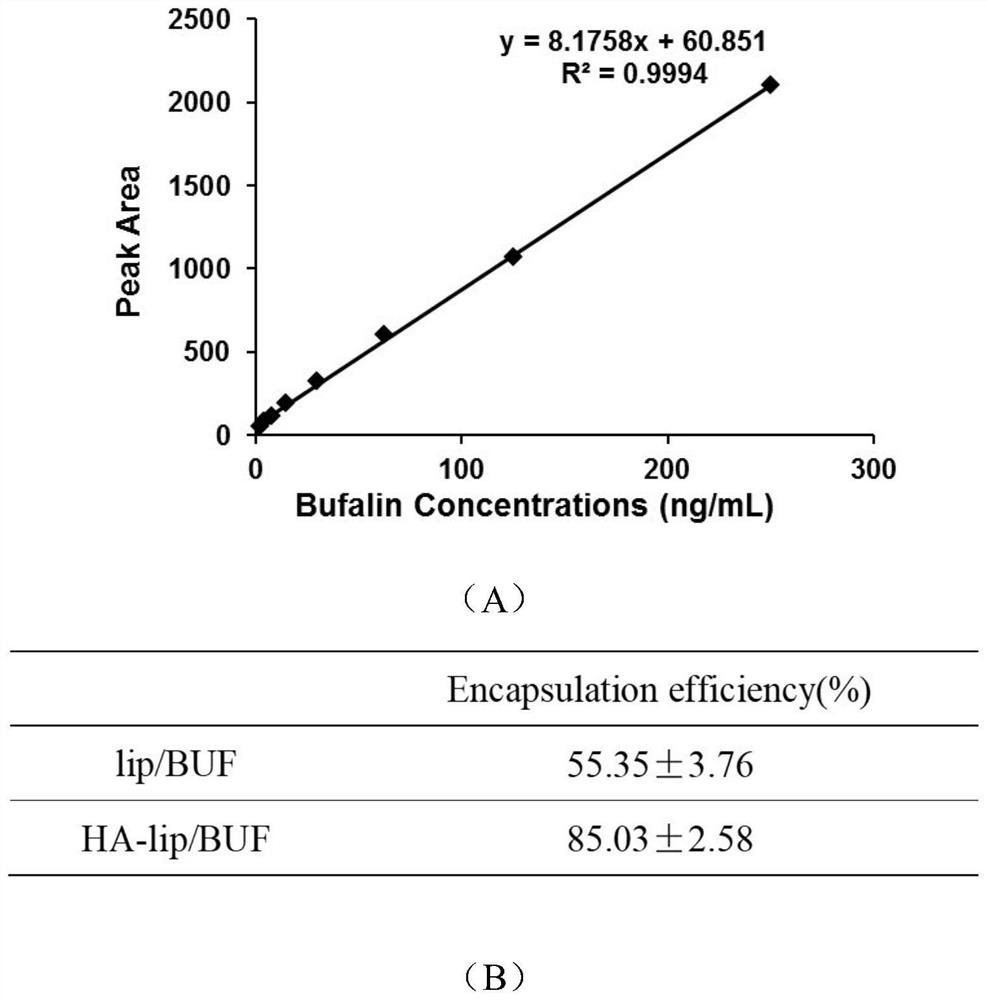
[0042] First, take 300 μL of liposomes and add 4 The upper part of the ultrafiltration centrifuge tube of u, 2000-5000r·min -1 Centrifuge for 30-40min, so that the liposomes are trapped on the upper part of the ultrafiltration tube, while the unencapsulated free drug is centrifuged to the bottom of the ultrafiltration tube. Take 100 μL of the upper liquid and add 900 μL of methanol, ultrasonically break the emulsion, centrifuge to get the supernatant, and use it for measurement. The result is as image 3 As shown, the standard curve was drawn with the concentration of bufalin as the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com