Dendropanax dentiger coumarin compound and application thereof to drug
A technology of ginseng and coumarins and compounds, applied in the field of medicine, can solve problems such as expensive and adverse drug reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Extraction and purification of three coumarin compounds in ginseng root
[0040] Take 10.0 kg of tree ginseng roots, heat and reflux three times with 100 kg of 70% ethanol for 3 hours each time, and concentrate the extract under reduced pressure to obtain 1.3 kg of extract. The extract was suspended in 1L of water, extracted successively with equal volumes of petroleum ether, ethyl acetate and n-butanol, and concentrated to dryness respectively. Take 216 g of n-butanol extract, mix the sample with silica gel (weight ratio 1:1), put it on a silica gel column (column size: 11*120cm), and mix it with dichloromethane-methanol (100:0→0:100, v / v) Gradient elution to obtain 9 fractions Frs. C1-C9. (1st to 9th fractions)
[0041] Dichloromethane-methanol 5:1 eluted fraction Fr.C7 (the seventh fraction 70.0 g) was subjected to octadecylsilane column chromatography (column specification: 4.2*40 cm), methanol-water (5%→30 %) gradient elution to obtain 3 fractions Fr...
Embodiment 2
[0043] Example 2: Structural identification of three coumarin compounds in ginseng
[0044]The compound of formula I, compound of formula II and compound of formula III separated in Example 1 were identified as three kinds of ginseng Soybean compounds. Wherein, the formula I structural compound is a new coumarin derivative that has not been reported in the literature, and the formula II structural compound and the formula III structural compound are known compounds (but the activity of treating rheumatoid arthritis and rheumatoid arthritis disease has not been see report).
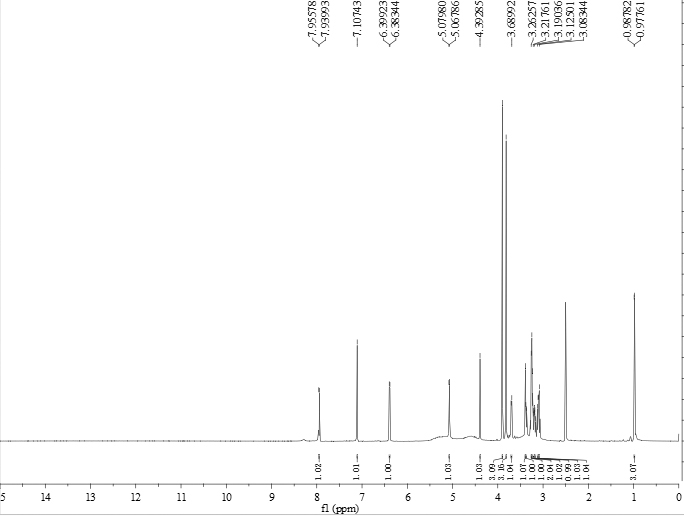
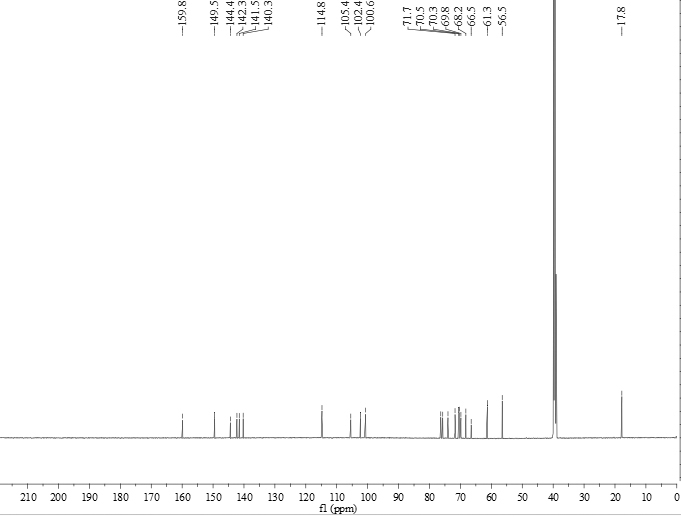
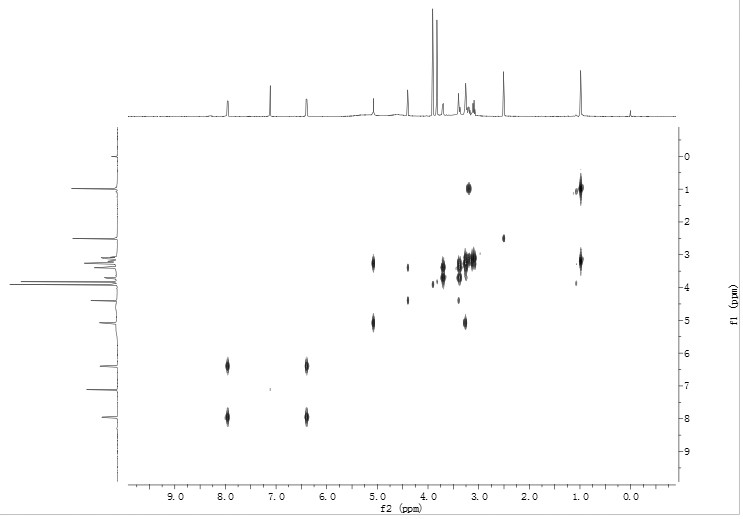
[0045] Wherein, the compound of formula I is light yellow powder. HR-ESI-MS gives the quasi-molecular ion peak m / z 531.17083 ([M+H] + , Calculated value: 531.17066), determine its molecular formula as C 23 h 31 o 14 . The hydrogen spectrum of formula I structure compound is as figure 1 As shown, (DMSO- d 6 , 600MHZ) there are 2 methoxy hydrogen signals δ H 3.90 and 3.82 (each, 3H, s, 8 / 6-CH 3 ...
Embodiment 3
[0053] Embodiment 3: Cytotoxicity evaluation experiment of coumarin compounds in ginseng to MH7A
[0054] Cell culture: MH7A cells (Guangdong Geneo Biotechnology Co., Ltd.) were subcultured for 5-10 passages, and the culture conditions were penicillin (final concentration: 100 U / mL), streptomycin (final concentration: 100 μg / mL), 10 High-glucose DMEM medium with % FBS, when the cells are confluent to 90%, discard the old medium, wash the cells twice with 2 ml PBS, add 2 mL of 0.25% (w / v) Trypsin-0.53 after discarding the PBS Mix the digestion solution with mM EDTA and observe it under a microscope for about 30 s. When the cells become round, quickly add 2 ml of complete medium to stop the digestion, and gently pipette to collect the cells. Centrifuge at 800 rpm at 4°C for 5 min, discard the supernatant, resuspend the cells with complete medium, culture in separate bottles, and change the medium every other day.
[0055] Cell viability assay: adjust MH7A cell density to 5 × 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com