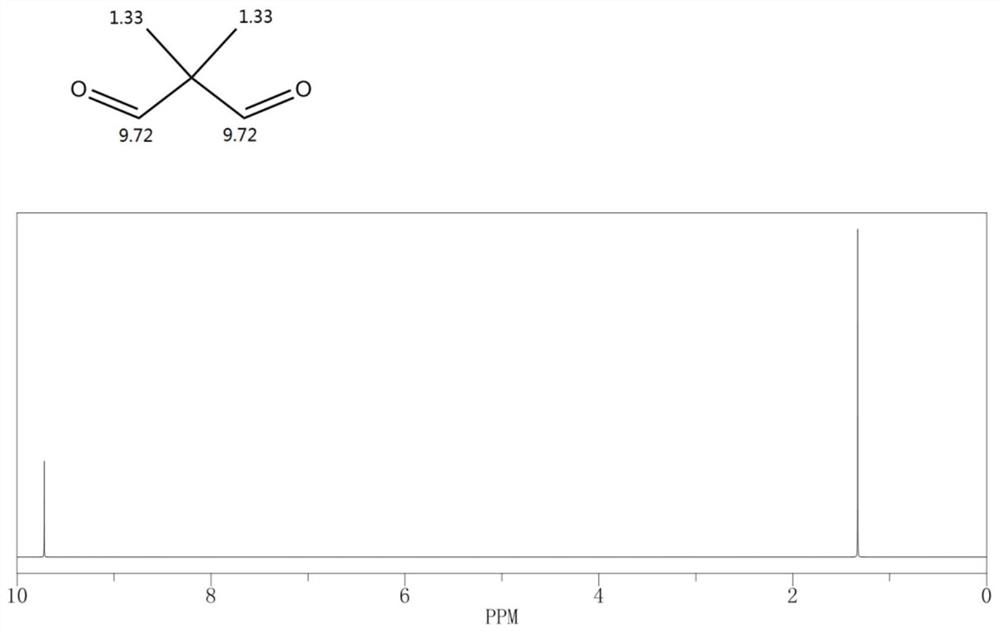
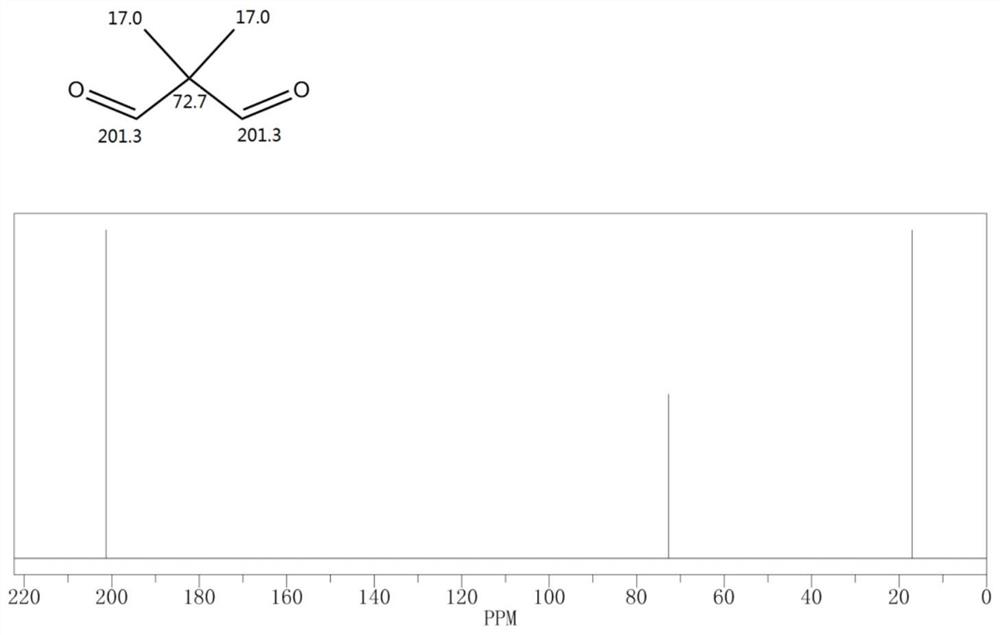
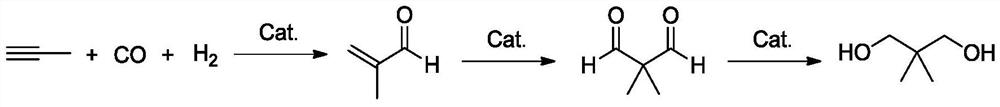
Carbonylation reaction catalyst composition and method for preparing neopentyl glycol
A technology of carbonylation reaction and catalyst, which is applied in the field of carbonylation reaction catalyst and preparation of neopentyl glycol, and can solve problems such as metal catalyst deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Add 0.35g of palladium acetate, 2.5g of triphenylphosphinepyrrole, and 1.0g of lanthanum trifluoromethanesulfonate to a 1L high-pressure stirred tank; Add 350g of propyne liquid and mix evenly; after the temperature of the mixed solution in the reactor is raised to 80°C, inject H 2 : CO molar ratio 1:1 syngas to a pressure of 5MPaG, stirring reaction, when the pressure in the reactor is reduced, the synthesis gas is replenished to 5MPaG, keep the temperature and pressure in the reactor constant, and react for 3h to obtain the reaction solution A; According to gas chromatography analysis, the conversion rate of propyne was 99.2%, and the selectivity of methacrolein was 98.6%.
[0056] Continue to raise the temperature of the reaction solution A to 130°C, feed the synthesis gas to a pressure of 20MPaG to start the reaction, and replenish the synthesis gas to 5MPaG when the pressure in the reactor drops, and obtain the reaction solution B after 10 hours of reaction; releas...
Embodiment 2
[0059] Add 0.11g rhodium carbonyl, 2.2g diphenylphosphine-naphthalenesulfonic acid, and 1.1g boron trifluoride to a 1L high-pressure stirred tank; Pass in 350g of propyne liquid and mix evenly; after raising the temperature of the mixed liquid in the reactor to 100°C, pass in H 2 :CO molar ratio of 1:1 synthesis gas to a pressure of 9MPaG, stirring and reacting, when the pressure in the reactor drops, the synthesis gas is replenished, the temperature and pressure in the reactor are kept constant, and the reaction solution A is obtained after 3.5 hours of reaction. According to gas chromatography analysis, the conversion rate of propyne is 99.5%; the selectivity of methacrolein is 98.3%.
[0060] Continue to raise the temperature of the reaction solution A to 120°C, feed the synthesis gas to a pressure of 16MPaG, and start the reaction. When the pressure in the reactor drops, the synthesis gas is replenished, and the reaction solution B is obtained after 12 hours of reaction; a...
Embodiment 3
[0063] Add 0.4 rhodium acetylacetonate, 1.85g triphenylphosphine-naphthalenesulfonic acid, and 0.65g copper bromide to a 1L high-pressure stirred tank; Add 350g of propyne liquid and mix evenly; after the temperature of the mixed solution in the reactor is raised to 65°C, inject H 2 :CO molar ratio of 1:1 synthesis gas to a pressure of 6MPaG, stirring and reacting, when the pressure in the reactor drops, the synthesis gas is replenished, the temperature and pressure in the reactor are kept constant, and the reaction solution A is obtained after 2 hours of reaction. According to gas chromatography analysis, the conversion rate of propyne is 99.3%; the selectivity of methacrolein is 98.8%.
[0064] Continue to raise the temperature of the reaction solution A to 145°C, feed the synthesis gas to the pressure of 22MPaG to start the reaction, and replenish the synthesis gas when the pressure in the reactor drops, and react for 14 hours to obtain the reaction solution B; after the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com