Synthesis of three-dimensional cadmium complex and application of three-dimensional cadmium complex as fluorescent probe
A technology of cadmium complexes and fluorescent probes, applied in cadmium organic compounds, fluorescence/phosphorescence, 2/12 group organic compounds without C-metal bonds, etc., to achieve high yield, high yield, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 complex:
[0025] 15.42mg of cadmium nitrate tetrahydrate (Cd(NO 3 ) 2 4H 2 O), 20.1mg of 3,3'-(1H,1'H-2,2'-biimidazole-1,1'-dimethylene)benzoic acid (H 2 L), 20.1 mg of 1,10-rophenanthroline (1,10-Phen) were dissolved in 2 mL of N, N-dimethylformamide and 1 mL of aqueous solution, ultrasonically oscillated for 5 minutes to mix well, and after mixing, put into a hot water The mixed solution was obtained in the polytetrafluoroethylene liner of the reaction kettle, and the mixed solution was baked at 100° C. for 48 hours. After the product was taken out, the solid was separated to obtain a colorless block crystal.
Embodiment 2
[0026] The structural characterization of embodiment 2 complex:
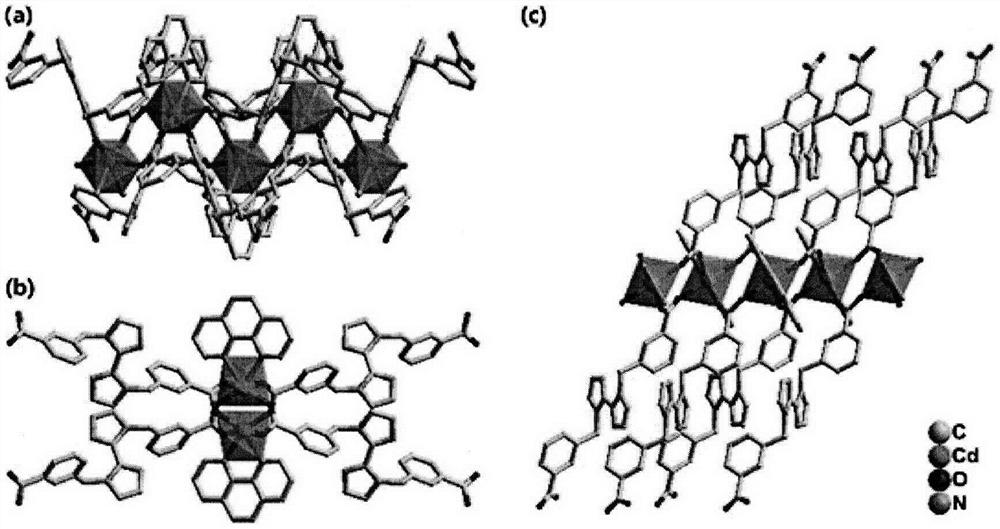
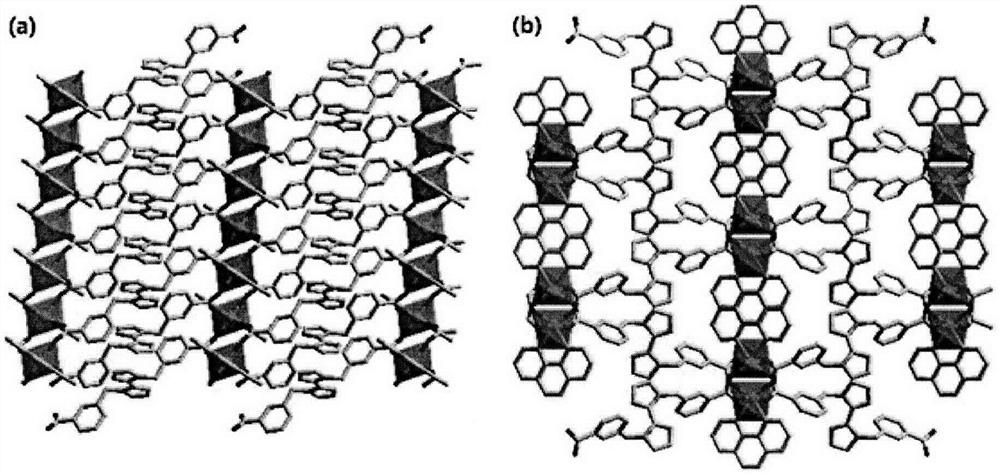
[0027] Use a microscope to select a single crystal of a suitable size, and use a Siemens (Bruker) SMART CCD diffractometer (graphite monochromator, Mo-Ka, ) to collect diffraction data. Diffraction data were corrected for absorption using the SADABS program. Data restoration and structure elucidation were done using the SAINT and SHELXTL programs, respectively. The coordinates of all non-hydrogen atoms were determined by the least squares method, and the positions of the hydrogen atoms were obtained by the theoretical hydrogenation method. The crystal structure was refined using the least squares method. Figure 1(a) and Figure 1(b) show the basic coordination and stacking modes. Some parameters of its crystallographic diffraction point data collection and structure refinement are shown in the table below.
[0028] Crystallographic data of the complexes in Table 1
[0029]
[0030]
[0031] R 1 =∑||...
Embodiment 3
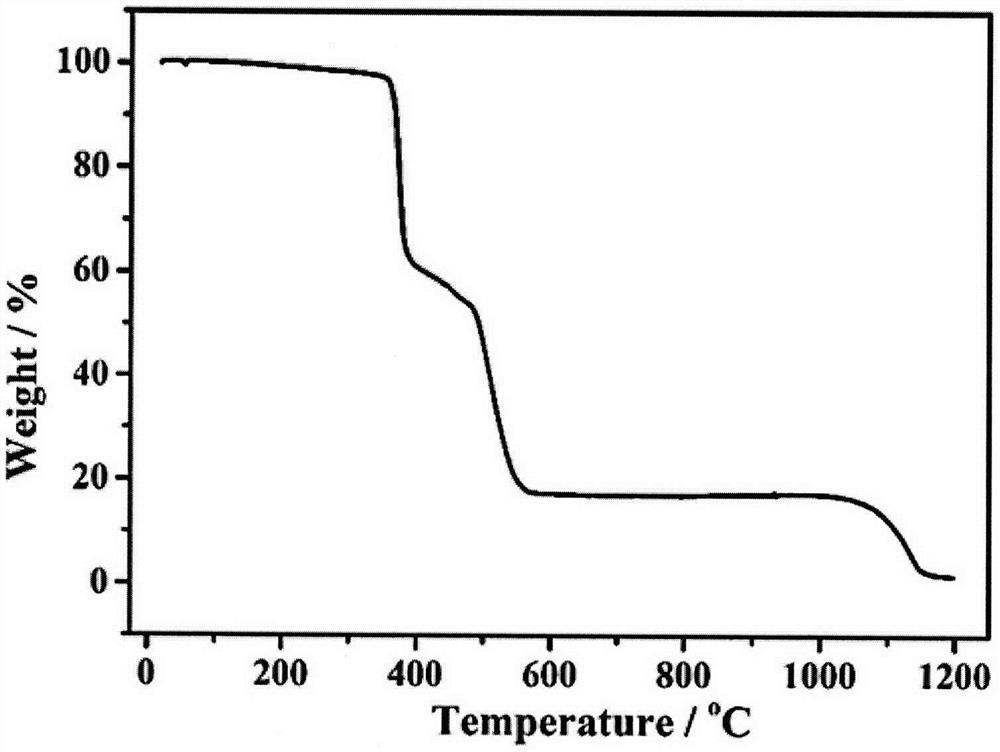
[0032] Embodiment 3: the thermogravimetric test of complex:
[0033] In order to explore the thermal stability of the cadmium complex of the present invention, a thermogravimetric test was carried out on its samples. The test conditions are: the protective gas is nitrogen at a flow rate of 20mL / min, the sweeping gas is air at a flow rate of 30mL / min, and the temperature is raised from room temperature to 1200°C at a rate of 10°C / min. Rapid weight loss within the range, which may be caused by the collapse of the crystal structure; when the temperature continues to rise, the remaining mass of the cadmium complex of the present invention remains basically unchanged, because relatively stable cadmium oxide is finally generated. The quality of the cadmium complex of the present invention does not change violently before 350 ° C, indicating that the thermal stability of the cadmium complex of the present invention is better ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com