Kit for detection of polymorphisms of Helicobacter pylori drug resistance gene by multiplex fluorescent PCR melting curve method
A technology of Helicobacter pylori and drug-resistant genes, applied in the field of biological detection, can solve the problems of reduced sensitivity, high detection cost, and long operation time, and achieve the effects of high amplification efficiency and sensitivity, short detection time, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
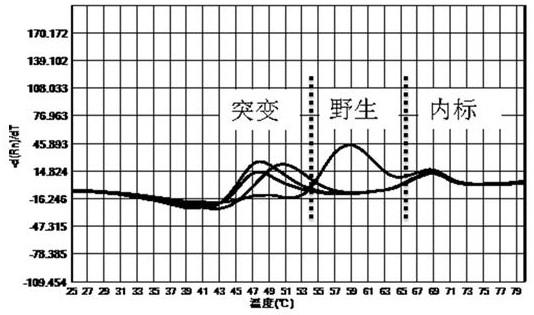
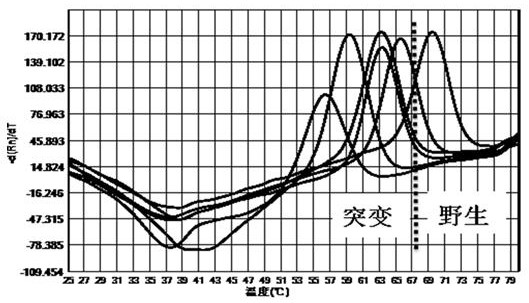
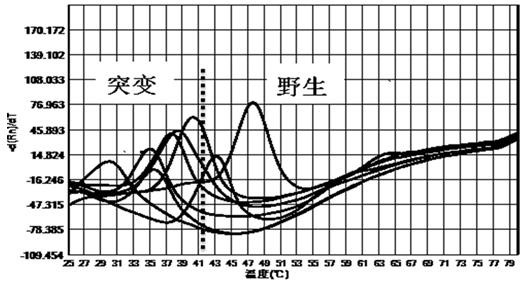
[0058] The kit of the invention is used for mutation detection of 260-261, 271-272 drug resistance sites of Helicobacter pylori gyrA gene, 2142, 2143 drug resistance sites of 23S rRNA gene, and 926-928 drug resistance sites of 16S rRNA gene.
[0059] 1. Design of primers and probes
[0060] According to the sequences of Helicobacter pylori gyrA gene, 23S rRNA base and 16S rRNA gene downloaded from NCBI, primer probes were designed around the drug resistance site to be detected according to the comparison results, and the primer design principles were followed, and the length was kept at 15-25bp, the primer Tm is kept at 53°C±2°C, and the design of the probe follows the design principle of the probe. The designed primers and probes were compared by BLAST to ensure the specificity of the primers and probes. The primer probe sequences are as follows:
[0061] The primer probe sequence for the resistance mutation site of Helicobacter pylori gyrA gene is as follows:
[0062] Ups...
Embodiment 2
[0108] Embodiment 2: Primer probe screening and detection ability comparison
[0109] The primer probe is the key raw material of the kit, and the optimization of the primer probe is more important. According to the sequences of the Helicobacter pylori gyrA gene, 23S rRNA base and 16S rRNA gene downloaded from NCBI, after comparison, the comparison results surround the Design primer probes for drug-resistant sites, follow the design principles of the primers, keep the length at 15-30bp, keep the Tm of the primers at 53°C±2°C, and follow the design principles of the probes. The designed primers and probes were compared by BLAST to ensure the specificity of the primers and probes.
[0110] The drug-resistant site sequence is a known sequence, and different kits design and optimize primers and probes around the same sequence, resulting in large differences in specificity and sensitivity between kits. The following describes the difference between the optimized primer probe of th...
Embodiment 3
[0126] Embodiment 3: clinical applicability verification
[0127] (1) According to the preparation method shown in Example 1, the relevant components of the kit were prepared and stored at -20°C for later use.
[0128] (2) About 300 cases of gastric mucosal tissue samples were collected from clinical units, and about 300 cases of clinical samples were extracted with the tissue extraction kit that has been filed by the company, and the purity and concentration of the extracted products were measured with Nanodrop2000, and the ratio of OD260 / OD280 of the samples All were between 1.6-2.1, and the nucleic acid concentration was >20ng / μL.
[0129] (3) According to the steps shown in Example 1, the steps of mixing the PCR reaction solution, adding samples, and operating on the machine were carried out. The detector needs to use an amplification instrument capable of multiple melting curve detection. The amplification instrument used in this experiment is Hongshi's slan.
[0130] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com