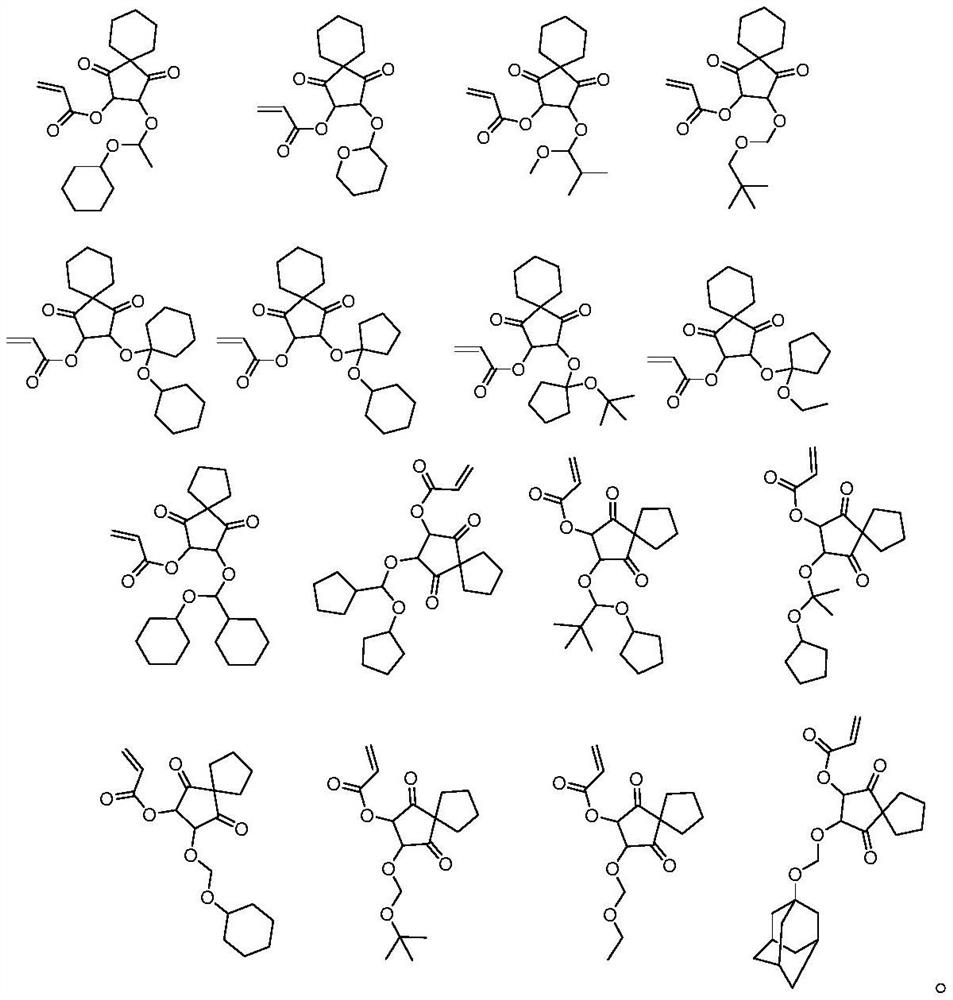
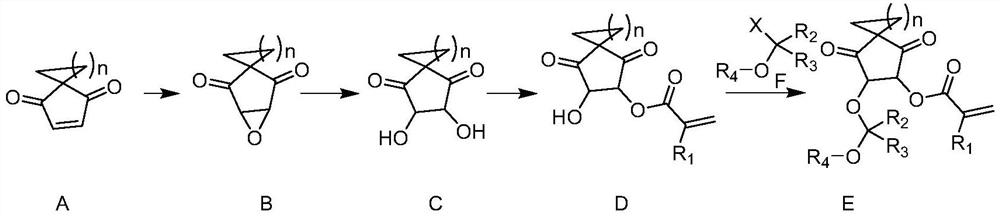
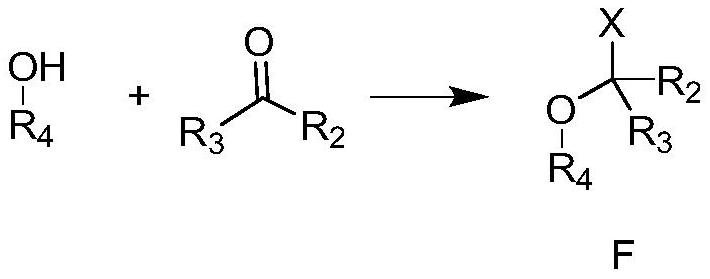
Photosensitive photoresist resin monomer containing polycyclic beta-ketone structure and synthesis method thereof
A technology of resin monomer and synthesis method, which is applied in the field of photosensitive resin monomer and its synthesis, can solve the problems of different solubility and poor polarity of polar developers, achieve good uniformity, large difference in solubility, and improve sensitivity and resolution rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add spiro[4.5]dec-2-ene-1,4-dione A (50g, 305mmol) into dichloromethane (1.2L), cool to 0°C in an ice-water bath, add m-chloroperoxybenzoic acid (62g , 359mmol), the reaction solution was raised to room temperature and stirred for 16 hours, filtered, the filtrate was neutralized by adding saturated sodium bicarbonate solution, the aqueous phase was extracted with dichloromethane (200mL×3), the organic phase was washed with saturated brine, and separated. After drying with anhydrous sodium sulfate and spin-drying in vacuo, the oily compound B (46.8 g, 260 mmol, 85.3%) was obtained.
[0026] Compound B (46.8g, 260mmol) was added to 3M sulfuric acid (500mL), heated to reflux and stirred for 16 hours, the reaction solution was cooled to 5 degrees Celsius, and sodium carbonate powder was added under stirring to adjust the pH to weak alkalinity, and the solvent was spin-dried , the solid was added into THF (500 mL) and stirred, the solid was removed by filtration, and the sol...
Embodiment 2
[0030] Compound D (10g, 40mmol) and 3,4-dihydro-2H-pyran (3.4g, 40mmol) were added to acetonitrile (100mL), methanesulfonic acid (0.5g, 5mmol) was added, and the reaction solution was stirred at room temperature React for 16 hours, add saturated sodium bicarbonate solution to quench the reaction, concentrate in vacuo to remove the acetonitrile solvent, add water (100 mL), extract the aqueous phase with ethyl acetate (100 mL×3), combine the organic phases, and wash the organic phases with saturated brine. Drying over anhydrous sodium sulfate gave the crude product, which was purified by distillation to obtain resin monomer E-2 (12.6 g, 37 mmol, 94.5%).
Embodiment 3
[0032] Add compound D (10g, 40mmol) to acetonitrile (200mL), add triethylamine (8g, 79mmol), cool to 0°C with ice water, slowly add 1-chloro-1-methoxy-2-methyl Propane (4.9g, 40mmol) in acetonitrile solution (80mL), raised to 50°C and stirred for 12 hours, then quenched the reaction by adding saturated sodium bicarbonate solution under ice water cooling, concentrated to remove acetonitrile, added ethyl acetate (200mL) and water (150mL), liquid separation, the aqueous phase was extracted three times with ethyl acetate (100mL×3), the organic phase was combined, the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried in vacuo to obtain the crude product, which was distilled and purified to obtain the resin Monomer E-3 (11.5 g, 34 mmol, 85.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com