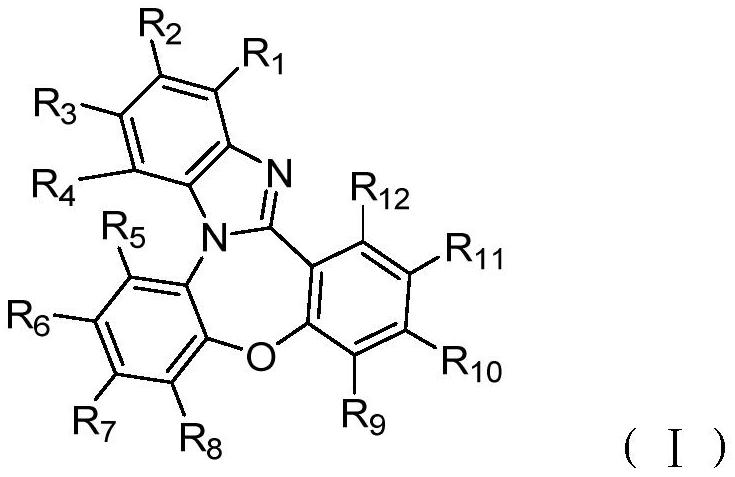
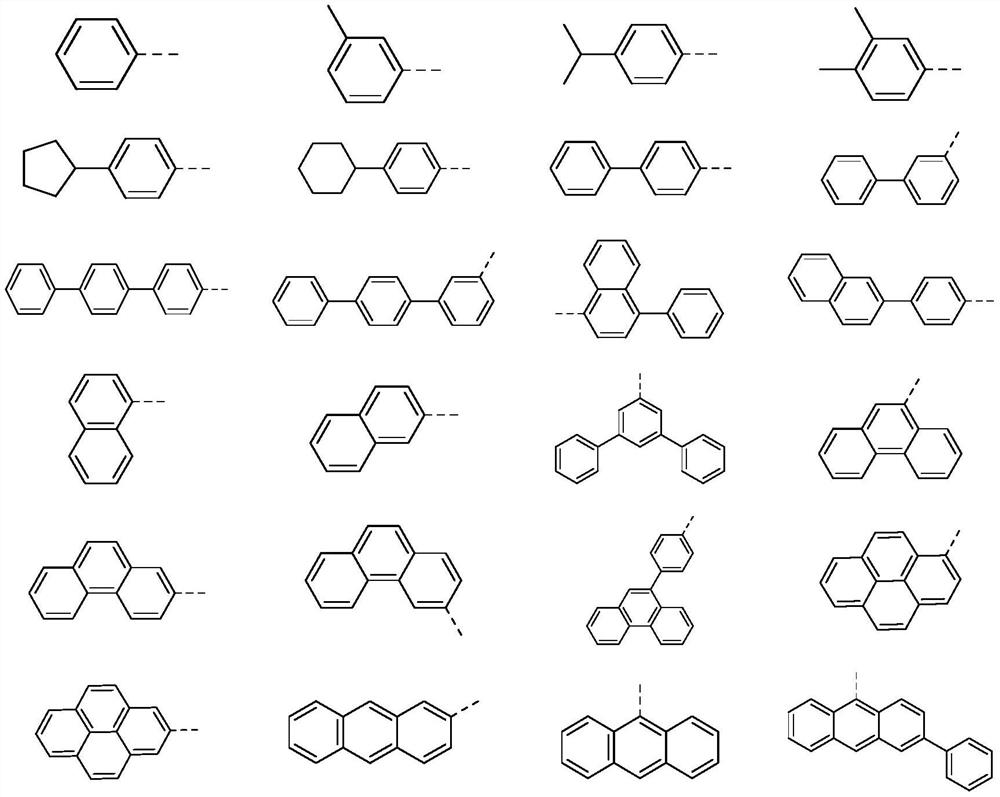
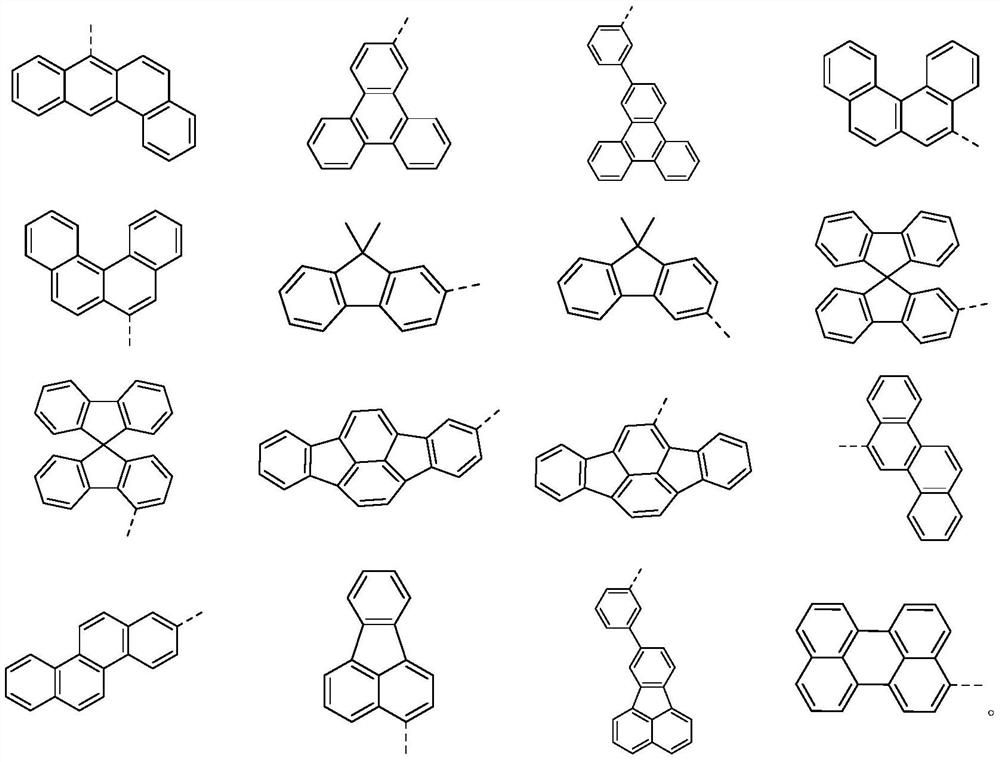
Compound with multi-heterocyclic structure and application thereof
A compound and heterocyclic technology, applied in the field of organic electroluminescent materials, can solve the problems of easy crystallization, few types of electron transport materials, and low triplet energy level of electron transport materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] According to the preparation method provided by the present invention, those skilled in the art can use known common means to realize, such as further selecting suitable catalysts, solvents, determining suitable reaction temperature, time, material ratio, etc., the present invention is not particularly limited to this . Unless otherwise specified, the solvents, catalysts, bases and other raw materials used in the preparation process can be synthesized through open commercial channels or methods known in the art.
[0053] Synthetic intermediates M1~M10
[0054] Synthesis of Intermediate M1
[0055]
[0056] The synthetic route is as follows:
[0057]
[0058] The specific operation steps are:
[0059] (1) In a 2L three-necked flask equipped with mechanical stirring, add 4-chloro-1-fluoro-2-nitrobenzene (17.5g, 0.1mol), 2-bromo-4-chloroaniline (30.8g, 0.15mol ), stirred, protected by argon, heated to 180°C, and kept warm for more than 30 hours. During the reacti...
Embodiment 1
[0110]
[0111] The synthetic route is as follows:
[0112]
[0113] The synthesis of compound I-1 comprises the following specific steps:
[0114] In a 1L three-neck flask, add M1 (38.8g, 0.1mol, purity 99%), phenylboronic acid (36.6g, 0.3mol, purity 99%), sodium carbonate (63.6g, 0.6mol), toluene 150mL, ethanol 150mL , water 150mL, the reaction system was replaced with nitrogen and then added Pd(PPh 3 ) 4 (11.5 g, 10 mmol). Heat to reflux for 3 hours to stop the reaction (the temperature in the system is about 78°C). The solvent was evaporated, extracted with dichloromethane, dried over anhydrous magnesium sulfate, filtered, petroleum ether / ethyl acetate (2:1) column chromatography, the solvent was spin-dried, ethyl acetate was beaten, and 38.9 g of a light yellow solid was obtained by filtration. The yield is about 76%.
[0115] Product MS (m / e): 512.19; Elemental analysis (C 37 h 24 N 2 O): theoretical value C: 86.69%, H: 4.72%, N: 5.46%; measured value C: 86...
Embodiment 2
[0117]
[0118] The synthetic route is as follows:
[0119]
[0120] Synthesis of compound I-8: replace M1 with M2, replace phenylboronic acid with 3,4-dimethylphenylboronic acid, select a suitable material ratio, and other raw materials and steps are the same as in Example 1 to obtain 36.4 g of a light yellow solid. The rate is about 74%.
[0121] Product MS (m / e): 492; Elemental Analysis (C 35 h 28 N 2 O): theoretical value C: 85.34%, H: 5.73%, N: 5.69%; measured value C: 85.14%, H: 5.93%, N: 5.39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com