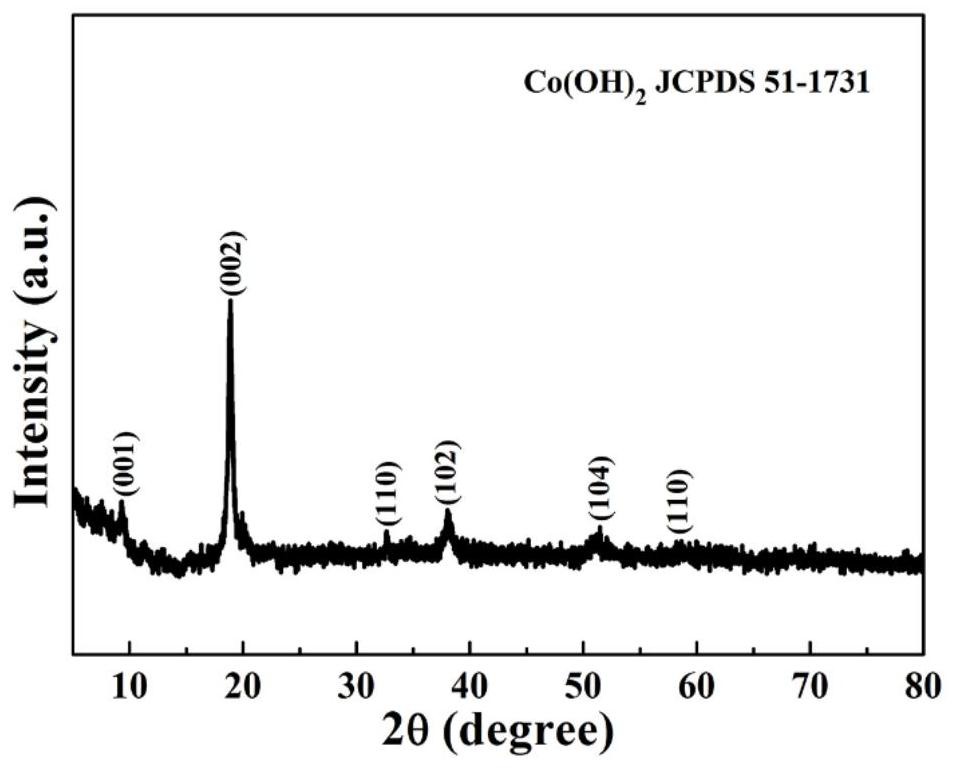
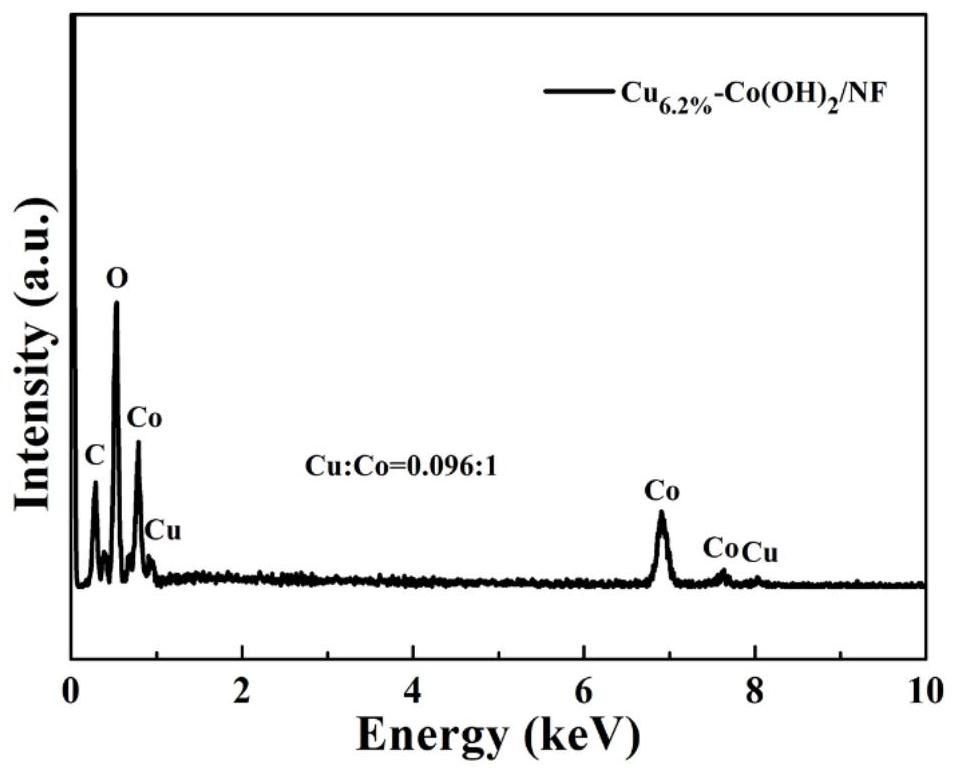
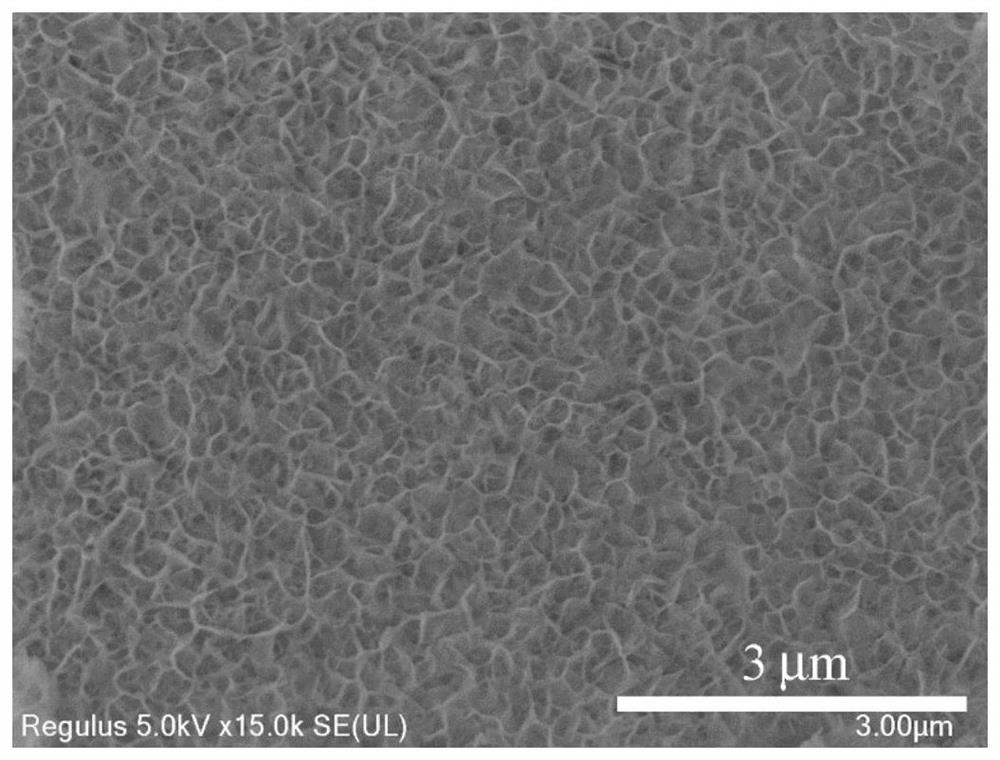
Cu-doped cobalt hydroxide nanosheet array structure material and preparation method and application thereof
A nanosheet array and structural material technology, applied in the field of Cu-doped cobalt hydroxide nanosheet array structure material and its preparation, can solve the problem that the catalytic activity needs to be further improved, so as to accelerate the interface charge transfer rate and increase the electrochemical performance. Active area, the effect of accelerating charge transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A Cu-doped Co(OH) 2 A method for preparing a nanosheet array structure material, comprising the following steps:
[0048] Soak 2×3cm foam nickel in 6M hydrochloric acid solution for 15 minutes, wash the foam nickel three times with deionized water and absolute ethanol, and dry naturally to obtain foam nickel with a clean surface. Accurately measure 20mL deionized water and 15mL methanol into a clean beaker, then weigh 0.2mmol Cu(NO 3 ) 2 ·3H 2 O, 2mmol Co(NO 3 ) 2 ·6H 2 O and 5 mmol of hexamethylenetetramine were added into the beaker to obtain a homogeneous solution. Transfer the solution to a 50mL polytetrafluoroethylene-lined stainless steel reaction kettle, insert the pre-treated nickel foam obliquely into the solution, seal it and react in an oven at 120°C for 6 hours, and cool it naturally to room temperature after the reaction is over. The nickel foam covering the sample was washed three times with deionized water and absolute ethanol, and then the nickel ...
Embodiment 2
[0057] Cu-doped Co(OH) 2 A method for preparing a nanosheet array structure material, comprising the following steps:
[0058] Accurately measure 20mL deionized water and 15mL methanol into a clean beaker, then weigh 0.1mmol or 0.3mmol Cu(NO 3 ) 2 ·3H 2 O, 2mmol Co(NO 3 ) 2 ·6H 2 O and 5mmol hexamethylenetetramine were added into the beaker and stirred evenly. Insert the dried nickel foam obliquely into a 50mL polytetrafluoroethylene-lined stainless steel reaction kettle, transfer the solution to the reaction kettle after the solution is fully dissolved, and react in an oven at 120°C for 6 hours after sealing. After the reaction was finished, it was naturally cooled to room temperature, and the nickel foam covering the sample was washed three times with deionized water and absolute ethanol, and then the nickel foam covering the sample was dried in an oven at 60°C for 8 hours. Cu(NO 3 ) 2 ·3H 2 When the amount of O added is 0.1 mmol, the Cu-doped Co(OH) with a Cu dopi...
Embodiment 3
[0063] A Cu-doped Co(OH) 2 Application of nanosheet array structure materials as catalysts for urea oxidation reaction (UOR).
[0064] The specific application method is: doping Co(OH) with Cu with an area of 0.5×0.5cm 2 The nanosheet array structure material was used as the working electrode, and the Pt wire and Ag / AgCl electrode were used as the counter electrode and reference electrode, respectively. The CHI760E electrochemical workstation was used for testing in 1.0M KOH and 0.33M urea electrolyte solutions. Co(OH) prepared on nickel foam and nickel foam supported by commercial Pt / C, respectively 2 Nanosheets were used as working electrodes, and their UOR properties were measured for comparison, Co(OH) 2 The preparation is to omit the Cu(NO in the raw material on the basis of embodiment 1 3 ) 2 ·3H 2 O prepared. Using linear sweep voltammetry (LSV) at 5.0mV s -1 Polarization curves were obtained at a scan rate of 90% and an ohmic compensation of 90%.
[0065] F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com