Method for removing heavy metal ions in high-salinity water by using hydrogen sulfide
A technology of heavy metal ions and high salt water, applied in chemical instruments and methods, water pollutants, chemical industry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
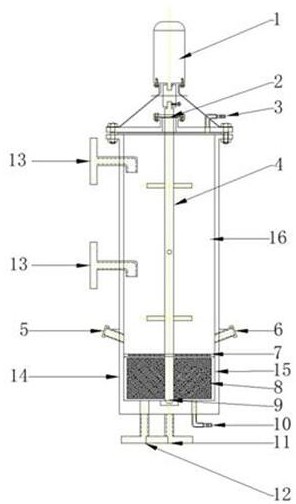
[0025] Reaction device: including a reaction kettle 14, a stirring paddle 4 penetrating through the kettle body is installed in the reactor body 14, and a sealing packing ring 2 is used to seal between the stirring paddle 4 and the reaction kettle body 14. A porous sieve plate 7 is arranged in the body of the reaction kettle, and the porous sieve plate divides the reaction kettle into a reaction zone 15 and a ripening zone 16 . The stirring paddle 4 is composed of three layers of evenly distributed flat-blade paddles and six porous flat-blade turbine paddles (the aperture is 0.5~5mm, and the stirring paddle material is titanium alloy, zirconium alloy, and nickel alloy materials that are resistant to acid and alkali corrosion.), And the flat-blade paddle is installed in the aging zone 16 of the reactor, and the porous flat-blade turbine paddle is installed in the reaction zone 15 of the reactor; the stirring paddle 4 is powered by the frequency conversion motor 1 installed on th...
Embodiment 2
[0036]Reaction device: with embodiment 1.
[0037] Reaction process: use the above device to conduct a continuous depth removal experiment of copper, nickel, lead and zinc in the mixed high-salt solution of sodium chloride and sodium sulfate with pH = 2.5. Set the peripheral speed of the stirring blades to 3.1 m / s, the molar ratio of hydrogen sulfide to heavy metal ions to 2.0, the temperature of the reaction liquid to 65°C, and the time it takes for the liquid to reach the outlet 13 to be 5.3 min. The specific operation process is:
[0038] (1) Close the liquid outlet valve and air inlet valve, open the liquid inlet valve and air outlet valve to ensure the airtightness of the reaction device;
[0039] (2) Pass the mixed high-salt solution into the reactor according to the designed liquid flow rate (100L / h), and feed the liquid while stirring;
[0040] (3) After the liquid in the kettle reaches 1 / 6 of the volume of the kettle, stop feeding the liquid, open the air inlet valv...
Embodiment 3
[0047] Reaction device: with embodiment 1.
[0048] Reaction process: use the above device to conduct a deep removal experiment for copper, nickel, lead, zinc and arsenic ions in the sodium chloride high-salt solution with pH=4.5. Set the peripheral speed of the stirring blade to 2.5m / s, the molar ratio of hydrogen sulfide to heavy metal ions to 2.0, the temperature of the reaction solution to 65°C, and set the time for the liquid to reach the outlet 13 to be 21 minutes. The specific operation process is:
[0049] (1) Close the liquid outlet valve and air inlet valve, open the liquid inlet valve and air outlet valve to ensure the airtightness of the reaction device;
[0050] (2) Pass the mixed high-salt solution into the reactor according to the designed liquid flow rate (25L / h), and feed the liquid while stirring;
[0051] (3) When the liquid in the kettle reaches 1 / 6 of the volume of the kettle, stop feeding the liquid, open the air inlet valve, and let in hydrogen sulfide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com