Method for preparing isobutylamido thiazolyl resorcinol
A technology based on thiazolyl resorcinol and isobutyramide, which is applied in the field of chemical synthesis, can solve the problems of unfriendly environment, many side reactions, and high cost, and achieve a simple synthesis process route, simple post-treatment, and few reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
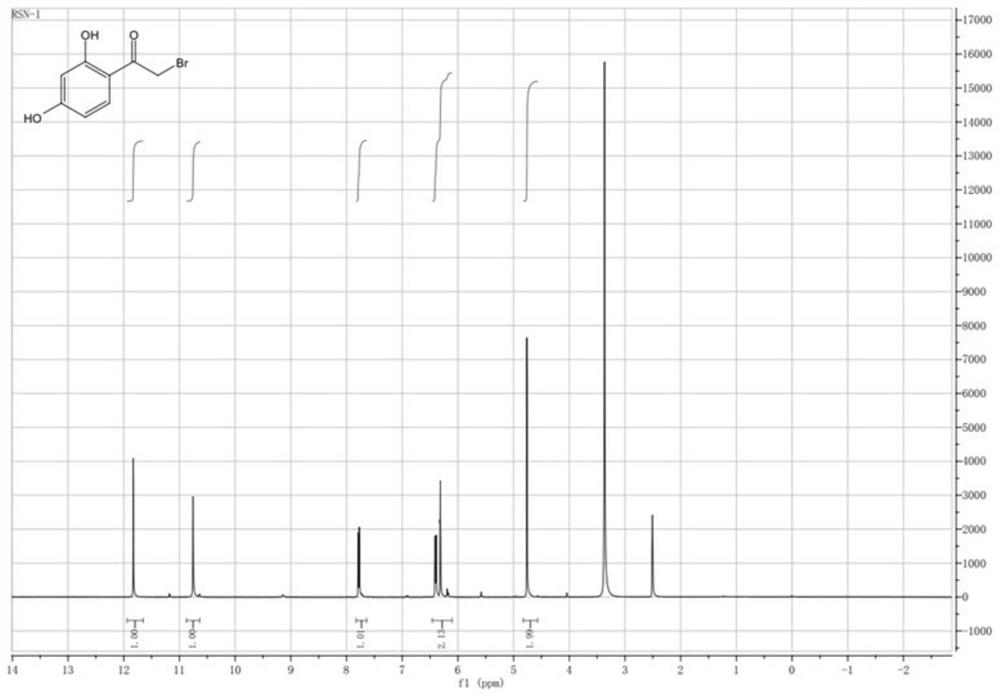
[0032] Embodiment 1: the synthesis of intermediate I
[0033] Weigh 2,220 grams of resorcinol, 2,100 grams of bromoacetic acid, and 5,500 mL of 48% boron trifluoride ether solution into a 10-liter reaction bottle, and slowly heat to 75°C (first heat to 65°C for 30 minutes, then heat up to 75°C °C to continue the reaction), the tail gas was absorbed with an aqueous solution of sodium hydroxide. After the reaction reaches 3 hours, trace the content of bromoacetic acid in the liquid phase. After it is less than 1%, cool to room temperature, slowly add 3000 ml of ice water dropwise, stir for 10 minutes, separate the liquid, dry the organic phase, and concentrate the organic phase to obtain about 2900 g of oil . The oil was separated by petroleum ether:ethyl acetate=50:1 column chromatography to obtain about 1800 g of off-white to light yellow solid with a purity of more than 98% and a yield of 51%.
Embodiment 2
[0034] Embodiment 2: the synthesis of intermediate II
[0035] Weigh 1,000 grams of isobutyryl chloride, 893 grams of thiourea, and 3 L of toluene, and add them to a 5-liter reaction bottle, reflux at 115°C for 3-4 hours, track the liquid phase, and absorb the tail gas with an aqueous solution of sodium hydroxide. Add 3 liters of water, distill the toluene, cool and crystallize. About 900 g of yellow crystalline solid was obtained by filtration, the purity was greater than 98%, and the yield was about 65%.
Embodiment 3
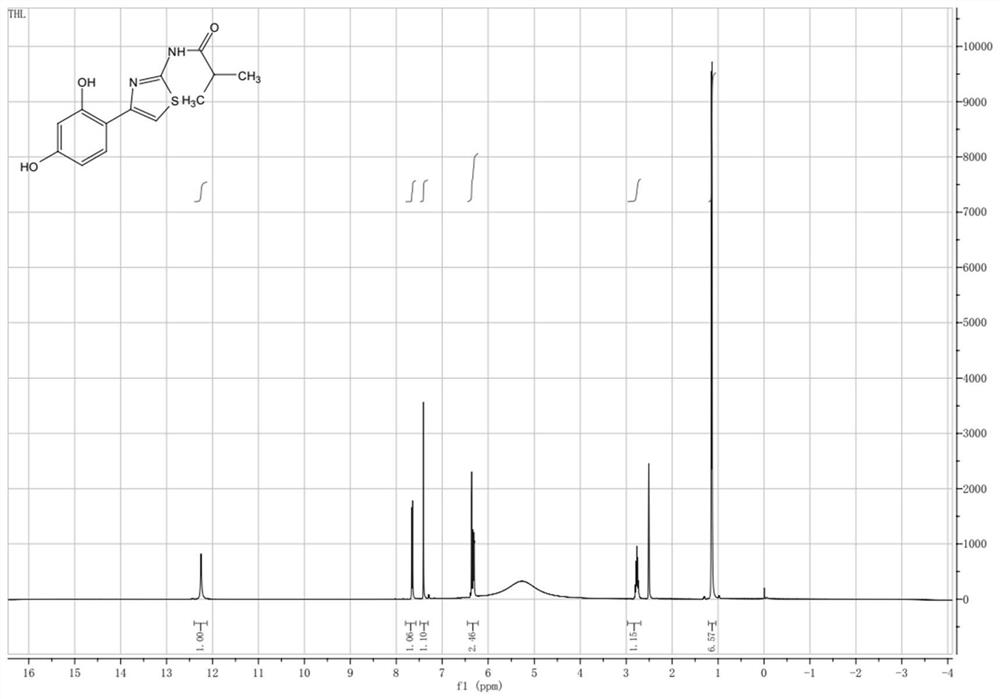
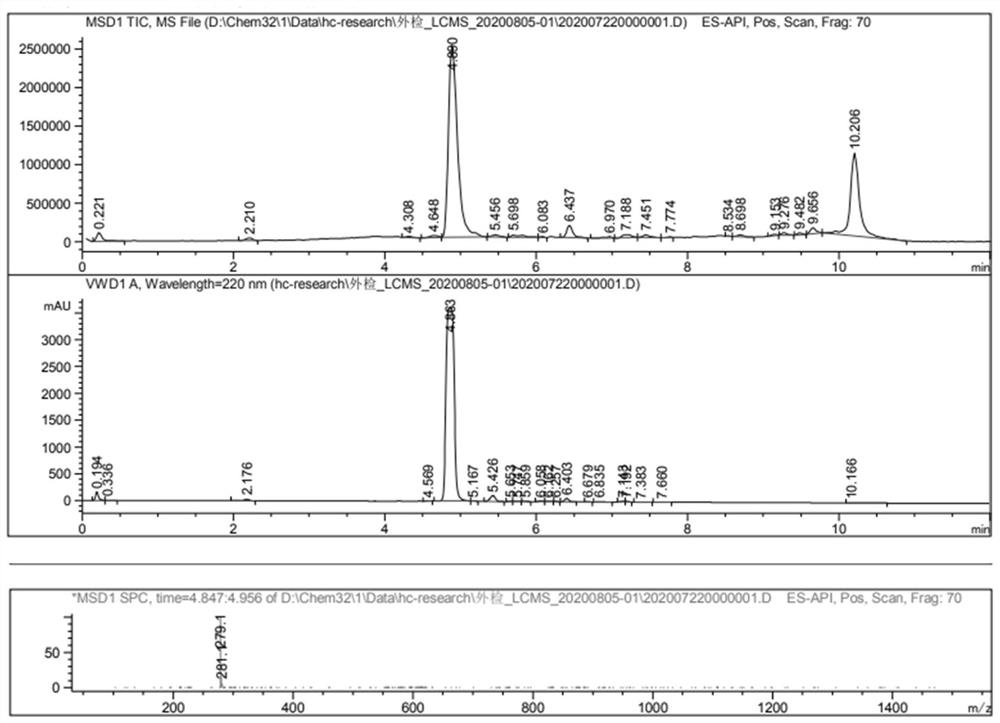
[0036] Embodiment 3: the synthesis of Thiamidol
[0037] Weigh 500 grams of Intermediate I, 333 grams of Intermediate II, 2.5 L of ethanol, and 285 grams of sodium bicarbonate, and add them to a 5-liter reaction bottle, and slowly raise the temperature to 80 ° C. After 30 minutes of reaction, follow the liquid phase, and the reaction is complete Finally, filter the inorganic salts, concentrate the organic matter, 1.5L ethyl acetate heat beating, and filter the product after cooling to obtain 530 grams of white to off-white solid with a purity greater than 99% and a yield of about 88%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com