Apoptotic protein fusion type anti-HER-2 single-chain antibody as well as preparation method and application thereof
A HER-2, single-chain antibody technology, applied in the direction of immunoglobulin, peptide/protein components, anti-enzyme immunoglobulin, etc., can solve the problems of poor stability of single-chain antibodies, cell death, high immunogenic side effects, etc. Achieve the effect of avoiding toxic side effects, high immunogenicity and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
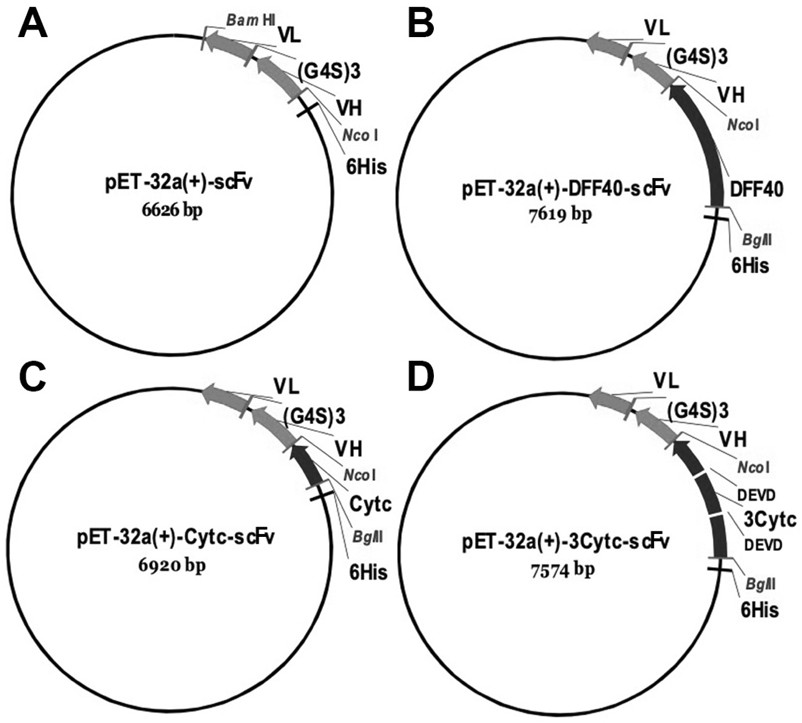
[0036] Example 1: Construction of pET-32a(+)-scFv recombinant plasmid
[0037] 1. Obtaining of scFv homologous recombination gene fragment of anti-HER-2 single chain antibody
[0038] 1.1 Heavy chain variable region VH, light chain variable region VL and flexible peptide (G 4 S) 3 sequence acquisition
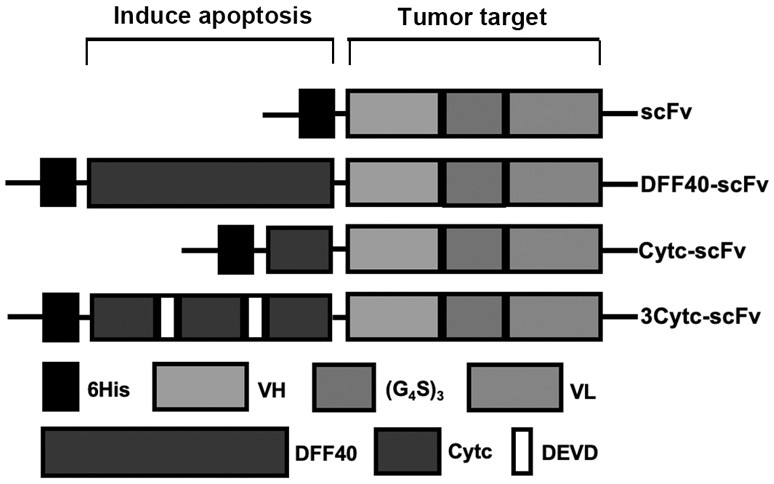
[0039] Through NCBI and Uniprot database search and literature search, compare the trastuzumab single-chain antibody sequence and linker sequence, and determine the amino acid sequence of the heavy chain variable region VH shown in SEQ ID NO.1, such as SEQ ID NO.2 The amino acid sequence of the light chain variable region VL shown and the flexible peptide (G) shown in SEQ ID NO.3 4 S) 3 amino acid sequence;
[0040] Heavy chain variable region VH sequence (SEQ ID NO.1): EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYIHWVRQAPGKGLEWVARIYPTNGYTRYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAMDYWGQGTLVTVSS;
[0041] Light chain variable region VL sequence (SEQ ID NO.2): DIQMTQSPSSLSASVGDRV...
Embodiment 2
[0054] Example 2: Construction of pET-32a(+)-Cytc-scFv, pET-32a(+)-3Cytc-scFv and pET-32a(+)-DFF40-scFv recombinant plasmids
[0055] 1. Experimental materials
[0056] 1. Primers required for PCR
[0057] Search and determine the cytochrome C (Cytc) amino acid sequence as shown in SEQ ID NO.6 and the cDNA sequence of coding described cytochrome C as shown in SEQ ID NO.7 by NCBI and Uniprot database search; Search by NCBI and Uniprot database Determine the amino acid sequence of DNA Fragmentation Factor 40 (DFF40) as shown in SEQ ID NO.8 and the cDNA sequence of encoding said DFF40 as shown in SEQ ID NO.9; According to the cDNA sequence of Cytc and DFF40 and pET-32a ( +) the sequence on the vector, design and synthesize PCR primers Cytc-F and Cytc-R for amplifying the gene sequence of the above-mentioned Cytc for homologous recombination with the pET-32a (+) vector; and amplify DFF40 for use with The PCR primers DFF40-F and DFF40-R of the gene sequence of vector homologous r...
Embodiment 3
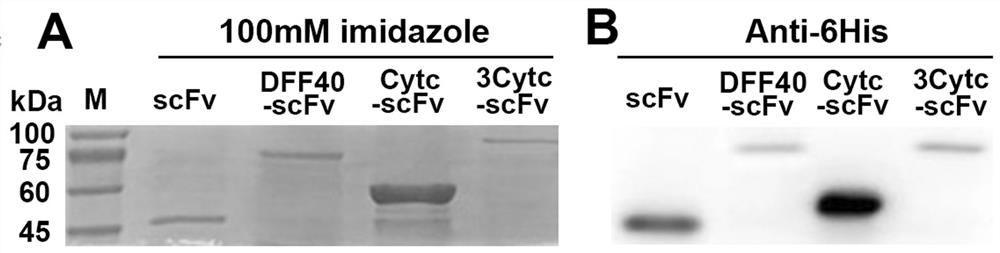
[0081] Example 3: Induced expression and identification of scFv, Cytc-scFv, 3Cytc-scFv and DFF40-scFv proteins
[0082] 1. Experimental materials
[0083] Escherichia coli expression strain competent cells BL21 (DE3) were purchased from Beijing Tiangen Biochemical Technology; Isopropyl-L-thio-β-D-galactopyranoside (IPTG) was purchased from China Aladdin; NI-NTA Resin was purchased from From Beijing Quanshijin Biotechnology;
[0084] 2. Experimental method
[0085] 1. Induced expression of scFv, Cytc–scFv, 3Cytc–scFv and DFF40-scFv proteins
[0086] The positive plasmid pET28a-scFv obtained in the above-mentioned Example 1 and the positive plasmid pET-32a(+)-Cytc-scFv, pET-32a(+)-3Cytc-scFv and pET-32a(+)- DFF40-scFv was transformed into Escherichia coli expression strain competent cells BL21(DE3), induced by IPTG and explored corresponding conditions to induce scFv, Cytc–scFv, 3Cytc–scFv and DFF40-scFv protein expression, and passed NI-NTA Resin Proteins purified by affini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com