Synthesis method of 3-sulfur-1-glycal compound
A synthesis method and technology of ensaccharides, applied in the field of synthesis of 3-thio-1-enose compounds, can solve the problems that the stereoselectivity of products needs to be improved, the regioselectivity needs to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Taking 3,4-O-carbonate galactenose and p-cresol as an example to optimize the experimental conditions, the details are as follows:
[0027]
[0028] Note: Unless otherwise stated, all experiments use 0.1mmol galactenose and 0.2mmol p-cresol, 10mol% cobalt catalyst, 20mol% monodentate phosphine ligand (or 10mol% bidentate phosphine ligand) in 2mL solvent 100 Schlenk tube reaction at ℃; isolated yield; regioselectivity and stereoselectivity >20:1 as measured by H NMR. XantPhos: 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene, DPPB: 1,4-bis(diphenylphosphine)butane, DPPF: 1,1'-bis( Diphenylphosphino) ferrocene.
[0029] The screening test of reaction conditions showed that the reaction conditions of cobalt tetrafluorobororide hydrate catalyst and Xantphos ligand in different solvents were different (entries 1-4), and Co(BF 4 ) 2 Catalyst, Xantphos ligand can get 3-thio-1-enose sugar in low yield (entries 1-2) in solvents such as dichloromethane and tetrah...
Embodiment 2
[0034]
[0035] Cobalt tetrafluoroborate hydrate Co(BF 4 ) 2 ·6H 2O (3.4mg, 0.01mmol), 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene (Xantphos, 5.8mg, 0.010mmol), zinc powder (0.01mmol) and 3, 4-O-carbonate galactenose 1 (0.1 mmol) was added to 2 mL of acetonitrile and m-cresol (0.2 mmol). Stir at 100 degrees Celsius, TLC detects the reaction progress, when the sugar raw material completely disappears, stop the reaction, extract and collect the organic phase, distill the solvent under reduced pressure to obtain the crude product, and then use petroleum ether / ethyl acetate solution as the mobile phase for column chromatography 4-Hydroxy-3-m-methylphenylthio-1-enose was obtained (93% yield).
Embodiment 3
[0037]
[0038] Cobalt tetrafluoroborate hydrate Co(BF 4 ) 2 ·6H 2 O, 3.4 mg, 0.01 mmol), 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene (Xantphos, 5.8 mg, 0.010 mmol), zinc powder (0.01 mmol) and 3, 4-O-carbonate galactenose 1 (0.1 mmol) was added to 2 mL of acetonitrile and o-cresol (0.2 mmol). Stir at 100-120 degrees Celsius, TLC to detect the reaction process, when the sugar raw material completely disappears, stop the reaction, extract and collect the organic phase, distill the solvent under reduced pressure to obtain the crude product, and then use petroleum ether / ethyl acetate solution as the mobile phase for column Chromatography afforded 4-hydroxy-3-o-methylphenylthio-1-enose (89% yield).
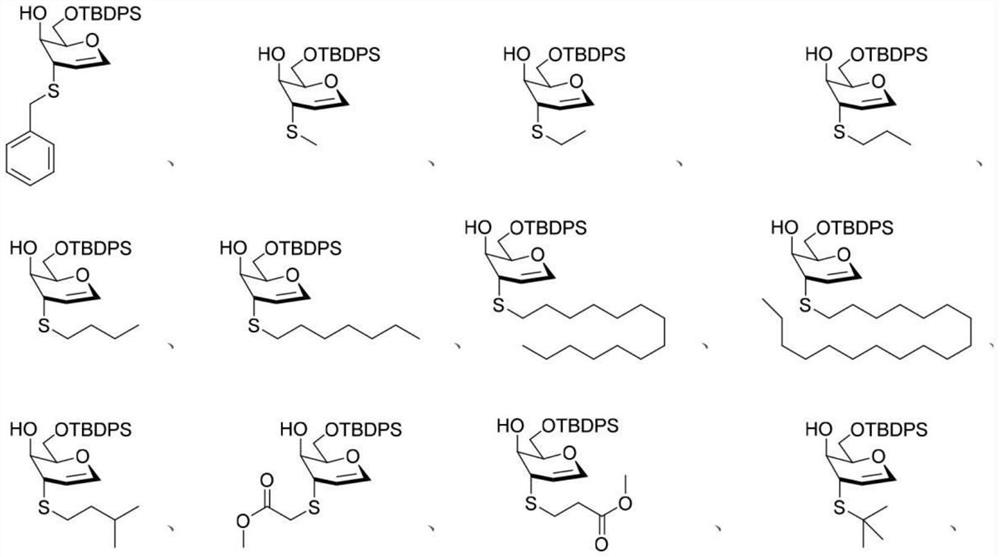
[0039] Substrate range
[0040] Mercaptans: preparation steps refer to Example 1
[0041]
[0042] Thiophenols: preparation steps reference example 1
[0043]
[0044] Aromatic heterocycles: preparation steps refer to Example 1
[0045]
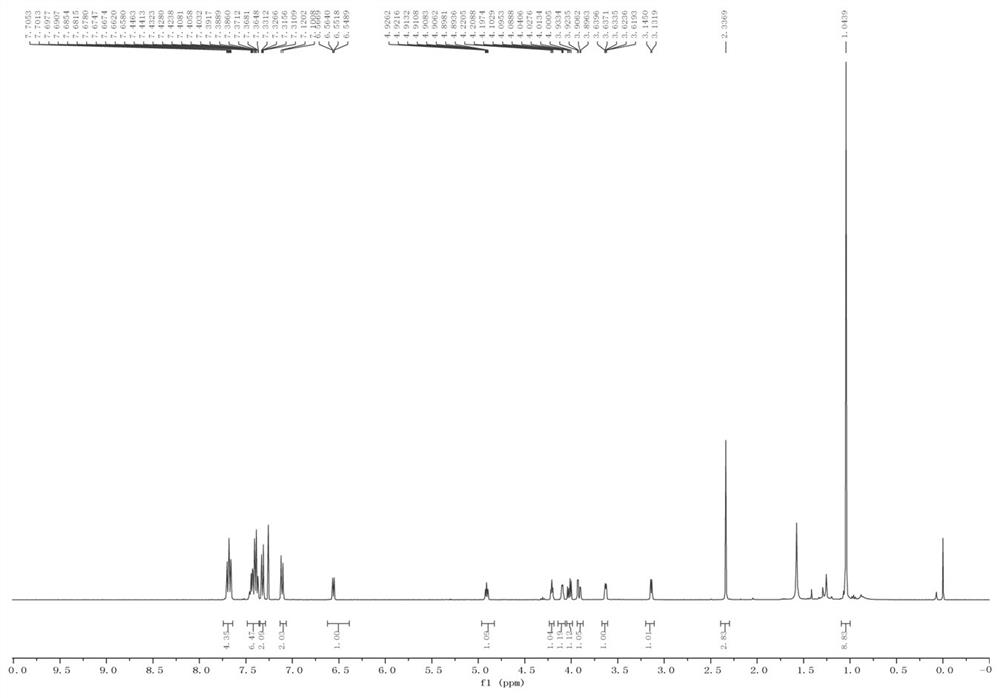
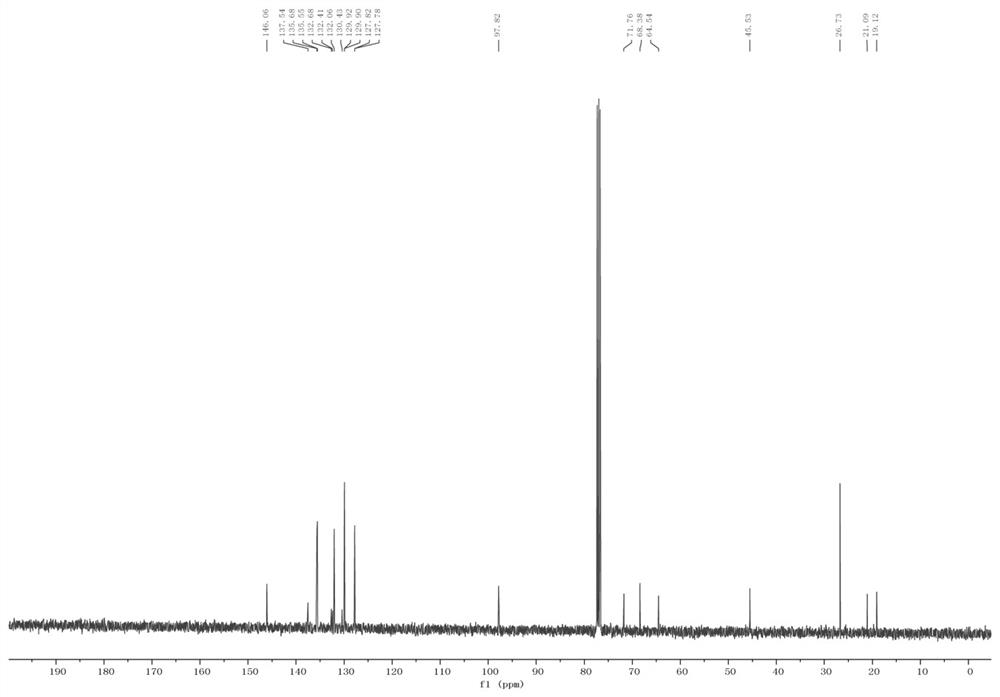
[0046] Spectral data
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com