Benzothiazole meroterpenoid compounds and derivatives thereof, and preparation method and application thereof
A technology for benzothiazole and compound, applied in the field of pharmaceutical compounds, can solve the problems of poor selectivity and high toxicity, and achieve the effects of simple steps, high product purity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
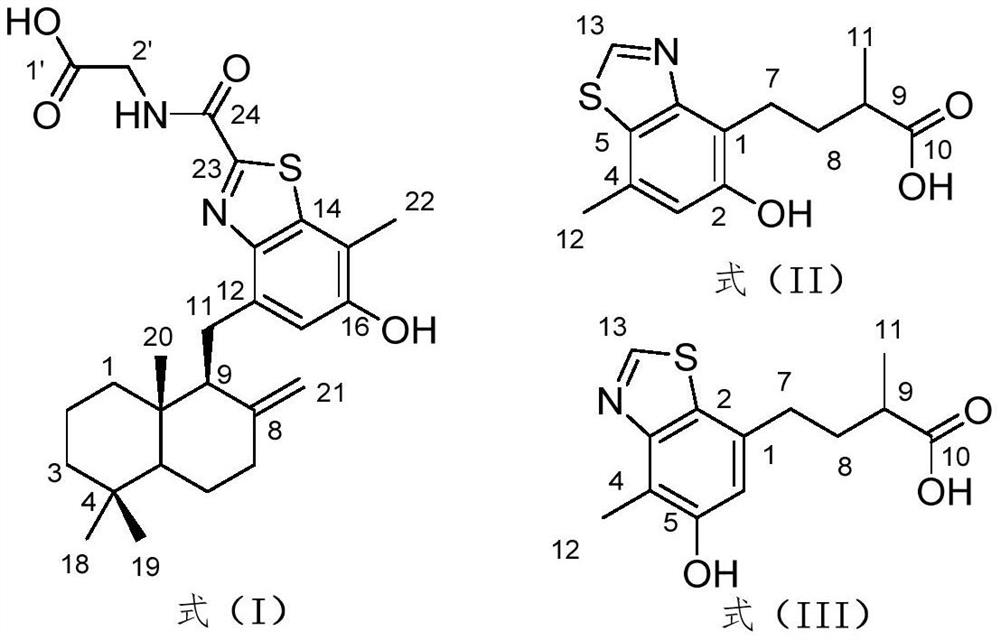
[0032] Example 1. Preparation of benzothiazole heteroterpenoids.
[0033] (1) Cultivate Penicillium allii-sativi (preserved in China Marine Microorganism Culture Collection Management Center, preservation number MCCC 3A00580) on a PDA plate at 25°C for 3 days; then connect the fresh mycelium to 400ml PDB containing After 24 hours, inoculate 10ml of seed solution into 1L Erlenmeyer flasks (100 bottles), each bottle has 80g of oats and 120ml of seawater with a salinity of 3%, and cultured statically for 30 days at 28°C;
[0034] (2) The fermented product obtained in step (1) is extracted three times with ethyl acetate, and the organic solvent is evaporated under reduced pressure to obtain an organic extract (200g); , dichloromethane and methanol were eluted; the dichloromethane layer was concentrated to obtain a crude extract (63.0 g);
[0035] (3) The crude extract obtained in step (2) was separated by normal phase silica gel column chromatography, and gradient elution was per...
Embodiment 2
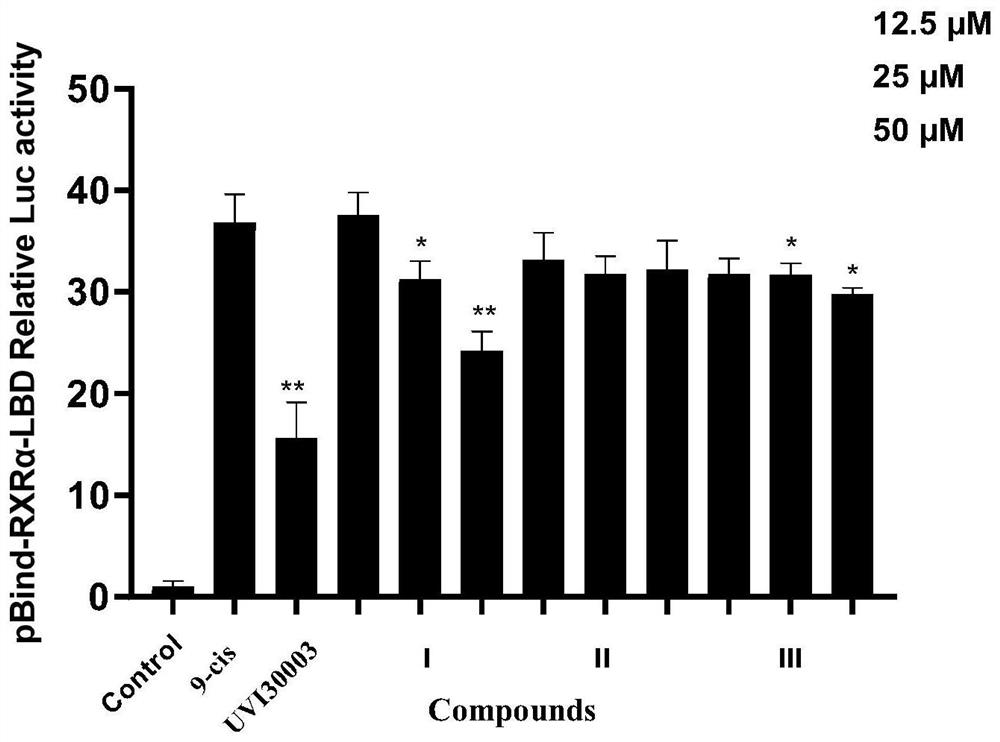
[0044] Example 2. Detection of RXRα dual reporter gene activity: Detect whether the compound can affect the transcriptional activity of RXRα, and preliminarily explore whether the compound may have a binding effect on RXRα.
[0045] In this example, a dual-luciferase reporter (DLR) detection system composed of firefly luciferase (Firefly luciferase, FL) reporter gene and Renilla luciferase (Rellina luciferase, RL) reporter gene was used. . The RL reporter gene was used as an internal control to normalize the measurements of the FL reporter gene. In cells lacking endogenous RXRα and its downstream signaling components, receptor RXRα and a reporter gene containing RXRα response elements are introduced by transfection, thereby simply simulating the transcriptional activation process of receptors in vivo.
[0046] This embodiment sets the following 3 groups:
[0047] Negative control group: equal amount of culture medium, 0.1% DMSO, containing cells, without adding compounds of ...
Embodiment 3
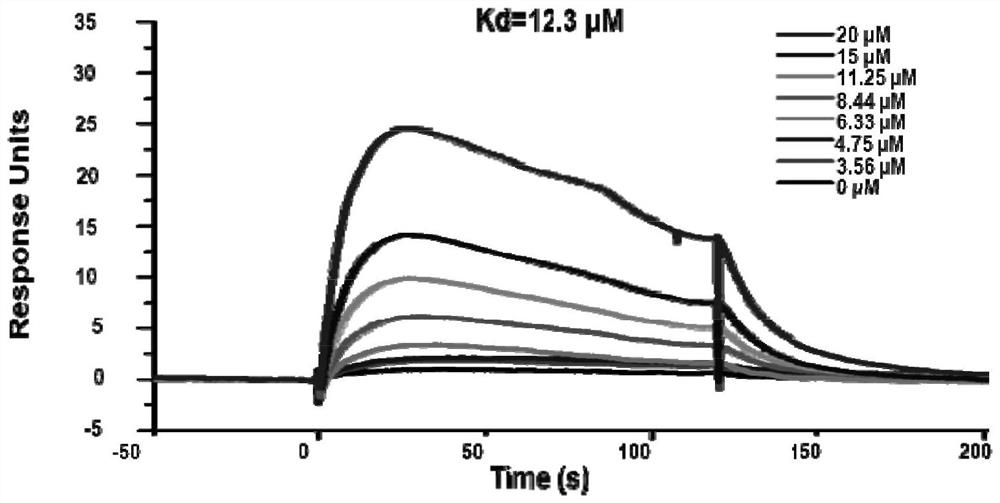
[0056] Example 3. Biacore experiment: evaluating whether compounds I-III bind directly to RXRα-LBD protein.
[0057] First, the purified nuclear receptor RXRα-LBD protein was coupled to Biacore's CM5 chip. Then the compounds to be tested were diluted with PBS to make solutions with different concentrations and then injected. When the sample to be measured flows over the surface of the chip, the combination of biomolecules causes the increase of the surface mass of the biosensor, resulting in a change in the refractive index. By monitoring the angle change of SPR, the kinetic binding and dissociation constants of the analyte can be automatically obtained. affinity and specificity etc. The BiacoreT200 detector can detect the interaction between the target protein and the sample in real time, and the binding strength is expressed in units of RU (response units, RU) (a change in the concentration of binding substances on the chip surface by 1pg / mm2 is defined as 1RU). We used Bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com