MAF-stu-13 material having ultra-microporous dia-a network topology as well as synthesis and application of MAF-stu-13 material
A technology of maf-stu-13 and network topology, applied in other chemical processes, ion exchange, organic chemistry, etc., can solve the problems of high cost, high energy consumption, complex process, etc., and achieve mild conditions, rapid mass synthesis, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthesis route of the synthesis of MAF-stu-13 in this embodiment is as follows:
[0064] 1. Solvothermal synthesis of MAF-stu-13:
[0065] A certain proportion of metal zinc salt: 0.1mmol, ligand H 2 L: 0.1mmol, DMF: 2mL, H 2 O: 1mL was placed in a 10mL hard glass tube, and the glass tube mouth was sealed with a water welder (oxygen hydrogen machine), and ultrasonically treated for 30min. Shake well and put it into a stainless steel box, heat it in an oven to 120°C and keep it at a constant temperature for 48 hours, then cool it down to room temperature at a rate of 5°C / h, open the tube and filter it, wash it twice with methanol, and filter it at room temperature. After natural drying, a large number of colorless needle-like crystals were obtained with a yield of about 76% (based on the ligand). After vacuum activation at 160°C for 12h, the MAF-stu-13 material with dia-a network topology was obtained.
[0066] 2. Rapid mass synthesis of MAF-stu-13:
[0067] We...
Embodiment 2
[0070] Analysis of crystallographic data of MAF-stu-13 material
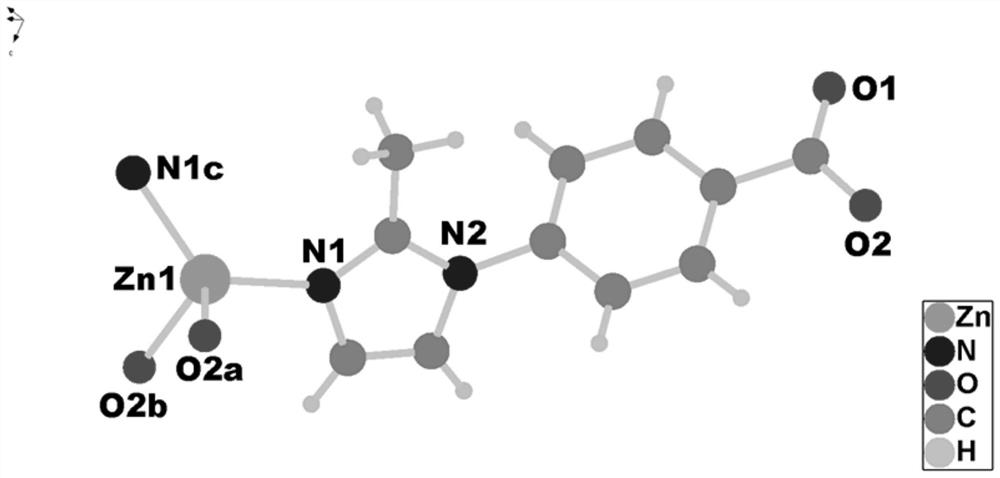
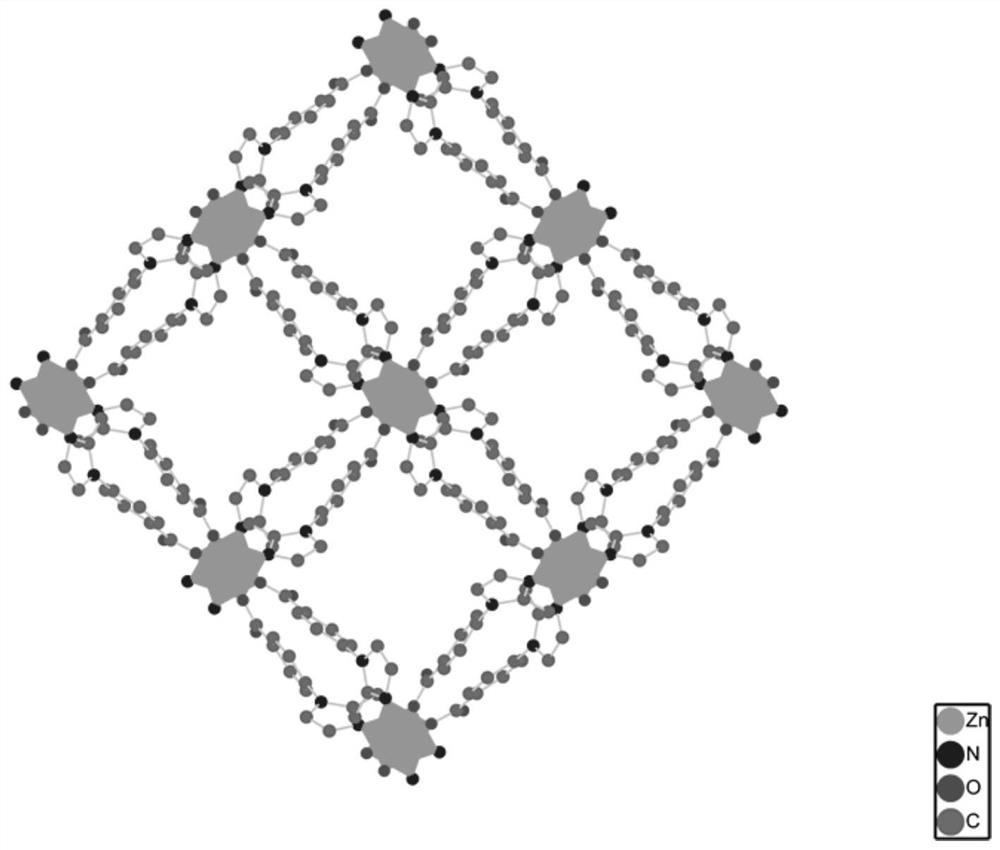
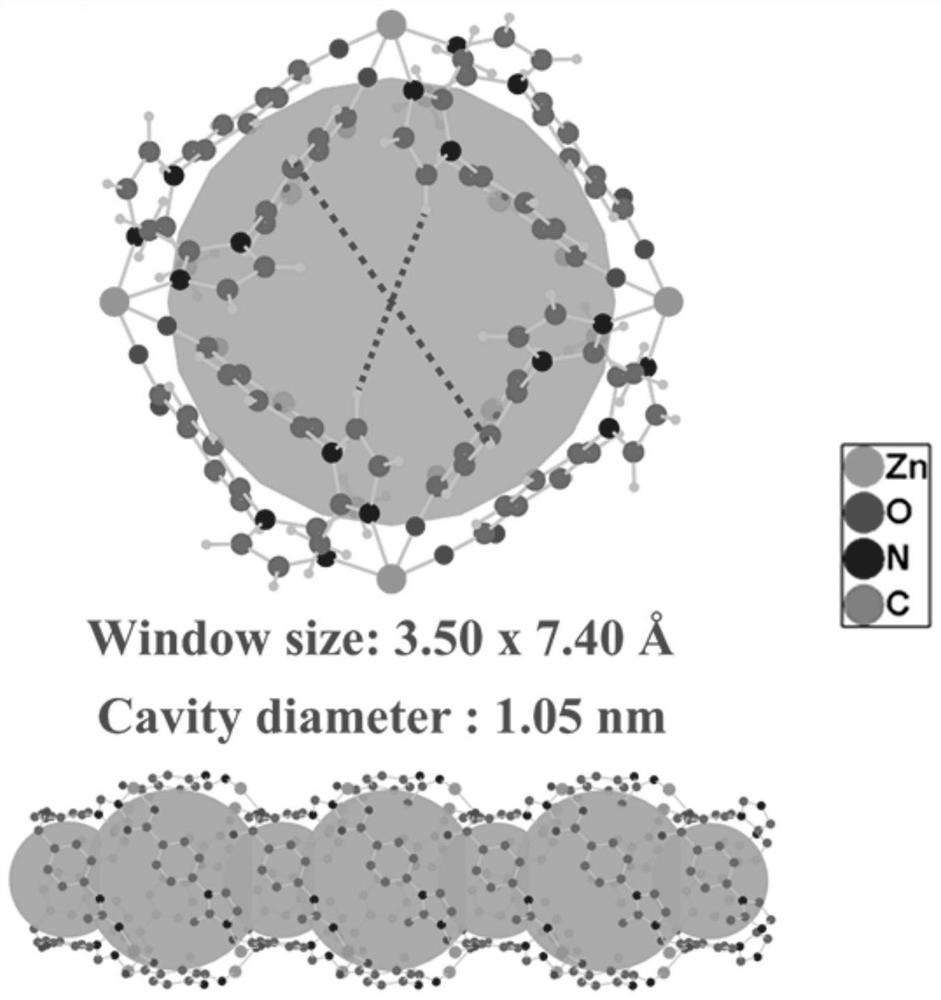
[0071] MAF-stu-13 material with ultramicroporous dia-a network topology, chemical formula {[Zn(MIBA) 2 ]} n , n is a non-zero natural number. MAF-stu-13 belongs to the tetragonal crystal system, P4nc space group, each asymmetric unit contains a crystallographically independent Zn(II) metal center, its occupancy rate is 1, and the coordination environment is as follows figure 1 shown. Zn(II) metal center adopts tetrahedral four-coordination mode The symmetry codes are: a-x-3 / 2, y+1 / 2, z+1 / 2; b x+1 / 2, -y-1 / 2, z+1 / 2; c-x-1, -y ,z. The Zn(II) metal center coordinates with the imidazole N and the carboxyl O in two independent ligands respectively, and one carboxyl O of the ligand does not participate in the coordination and is exposed in the channel (such as figure 2 shown). MAF-stu-13 is a quadruple interspersed structure (such as Figure 4 As shown), there is a one-dimensional channel in the c-axis dire...
Embodiment 3
[0077] Characterization of thermal stability and chemical stability of MAF-stu-13 material.
[0078] A certain quality of MAF-stu-13 crystal was weighed, and thermal stability, water stability, pH stability and organic solvent stability were tested respectively. The PXRD spectrum showed that MAF-stu-13 had excellent thermal stability and chemical stability.
[0079] In the TGA curve (such as Figure 7 shown), the weight loss platform of MAF-stu-13 before 80°C indicated that the guest molecules in the framework were lost and remained stable at 350°C, and then when the temperature continued to rise to 800°C, the weight loss reached 65%, and the MAF-stu-13 framework was completely Decomposition, the residue is ZnO. By variable temperature PXRD spectrum (such as Figure 8 (shown) shows that MAF-stu-13 crystals can remain stable below 350 °C. In addition, MAF-stu-13 can be maintained in aqueous solution for one month and heated in a boiling water bath for 7 days, and its struct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com