Luteolin-4'-o-(6"-o-acetyl)-β-d-glucoside and its preparation method and application
A technology of luteolin and glucoside, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problem of few box fruit vines, and the effective chemical composition and action mechanism of box fruit vines are not clear and cannot be realized. Large-scale extraction and application, etc., to achieve the effect of high extraction rate, strong anti-inflammatory activity, and inhibition of NO release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of luteolin-4'-O-(6"-O-acetyl)-β-D glucoside:
[0037] Get 2.5kg of box fruit vine coarse powder, reflux extraction with 10 times of 95% and 70% ethanol successively for three times, concentrate under reduced pressure to obtain 500g of total extract, suspend with water, and use petroleum ether, ethyl acetate, n-butanol successively The extraction was repeated, and the solvent was recovered to obtain 40 g of petroleum ether, 48 g of ethyl acetate, and 200 g of n-butanol.
[0038] Take 200g of n-butanol part extract, separate with macroporous resin column chromatography, water-ethanol (100:0→70:30→50:50→30:70→10:90→0:100) gradient elution, get Fractions D1-D5 and D2 were separated by MCI reverse-phase column chromatography, using water-methanol (100:0→80:20→70:30→60:40→50:50→30:70→0:100 ) for gradient elution to obtain fractions E1-E7, and E3 was subjected to semi-preparative high performance liquid chromatography (water-methanol=44:56) to obtain 3.2 mg of th...
Embodiment 2
[0040] Structural identification of luteolin-4′-O-(6”-O-acetyl)-β-D glucoside:
[0041] The compound product of Example 1 is a yellow powder, insoluble in petroleum ether and chloroform, and easily soluble in methanol. Hydrochloric acid-magnesium powder reaction and Molish reaction were all positive, presumably flavonoid glycosides.
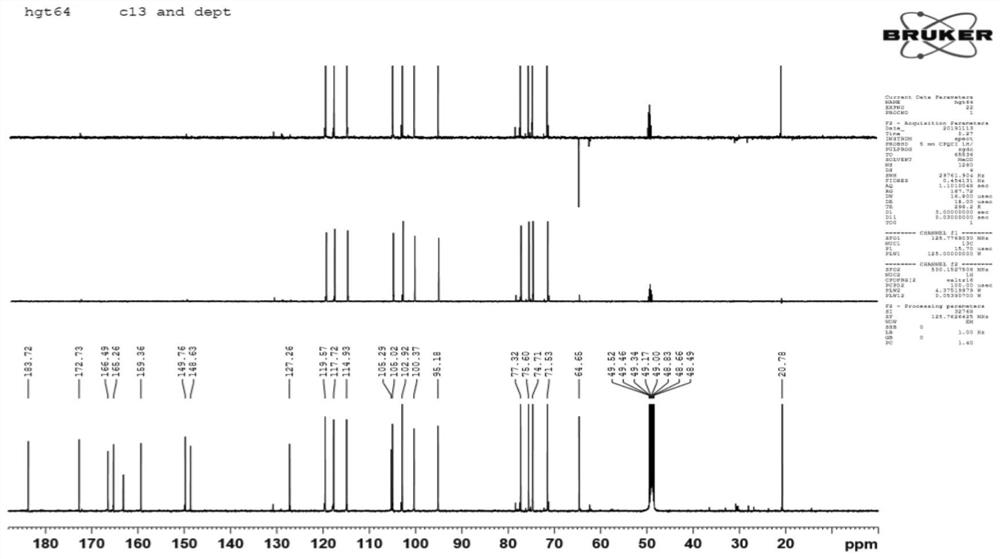
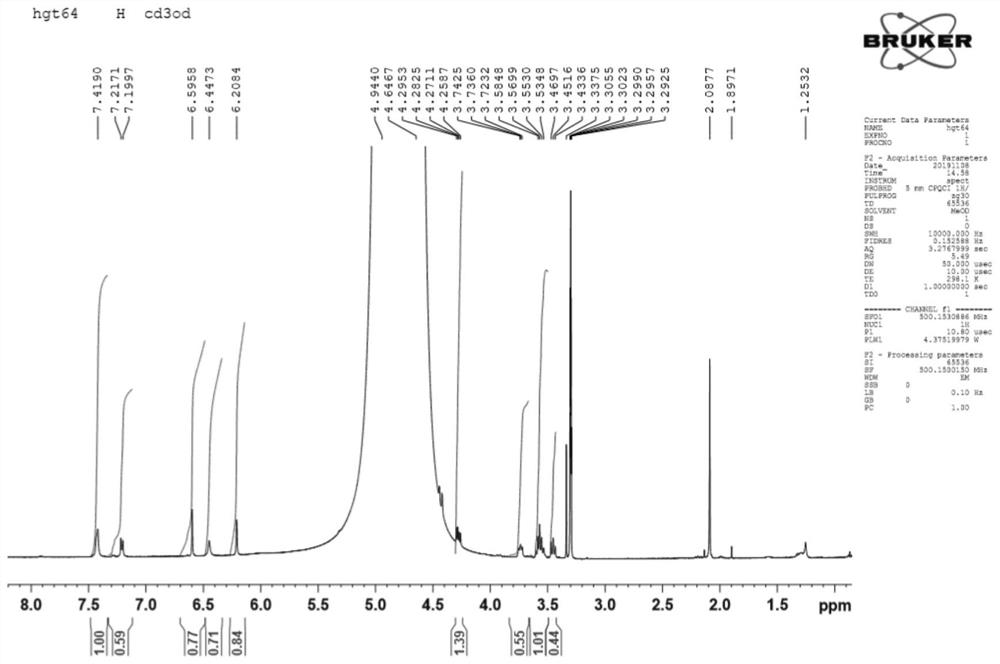
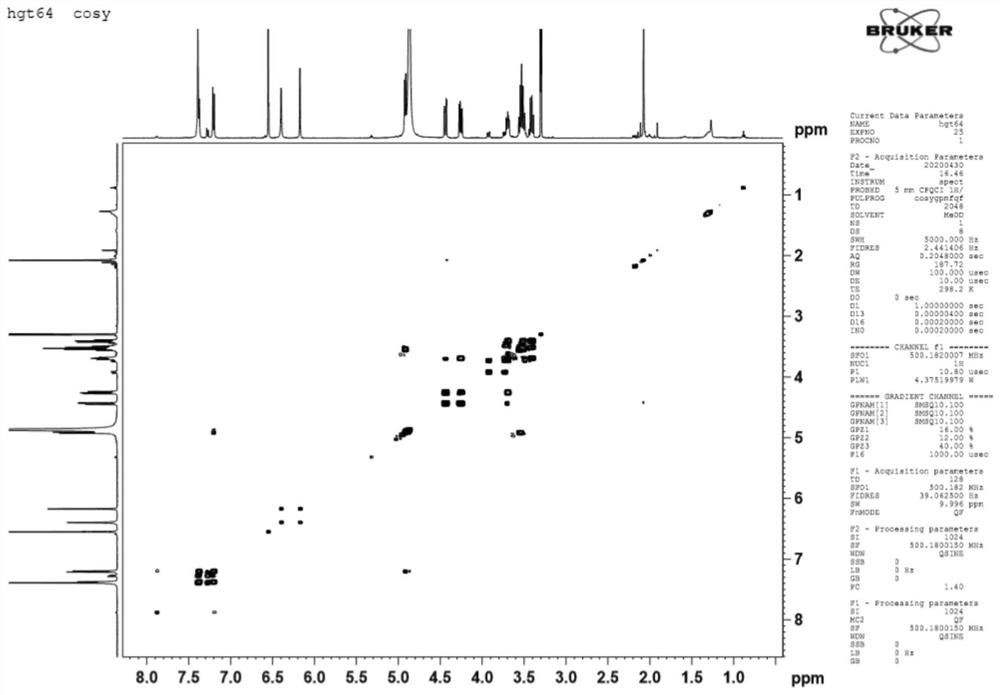
[0042] HR-ESI-MS (m / z): 513.1005[M+Na]+(clacd for C 23 h 22 o 12 Na,513.1009), comprehensive 1 H-NMR and 13 C-NMR can speculate that the molecular formula of the compound is C 23 h 22 o 12 , with a molecular weight of 490 and a degree of unsaturation of 13.
[0043] 1 H-NMR (500MHz, CD 3 OD) δ: 7.40 (1H, d, J = 2.1 Hz), 7.39 (1H, dd, J = 8.4, 2.1 Hz), 7.22 (1H, d, J = 8.5 Hz) indicates the existence of an ABX system. 6.56 (1H, s) is H-3, 6.41 (1H, brs, H-8), 6.19 (1H, d, J=1.8Hz) shows that ring A is 5,7-disubstituted. δ4.93(1H,brs,H-1”) is the signal of sugar anogroup; 2.09(3H,s) is -COCH 3 Signal.
[0044] 13 C-NMR and DEPT spect...
Embodiment 3
[0053] Anti-inflammatory activity of luteolin-4′-O-(6”-O-acetyl)-β-D glucoside as a drug
[0054] 1. Using the CCK8 method to determine the effect of the luteolin-4'-O-(6"-O-acetyl)-β-D glucoside sample of Example 1 on cell activity
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com