Subunit fusion protein tG on surface of rabies virus, and preparation method and application of subunit fusion protein tG
A fusion protein, rabies virus technology, applied in biochemical equipment and methods, viruses, viral peptides, etc., can solve the problems of inability to large-scale industrialization, inability to produce applications, and high production costs, achieving batch-to-batch stability and easy large-scale production. The effect of easy production and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 G protein expression and design
[0045] 1.1 Selection of rabies virus G protein
[0046] The rabies virus surface envelope protein G protein is composed of an extracellular region whose antigenic site is located in the extracellular 1M-455N amino acids, wherein 1-19 amino acids are signal peptides, and 20-445 amino acids are mature amino acid extracellular regions. G protein is a homotrimer in structure. Although G protein, as an important protective antigen, has been deeply studied and reported as early as the 1980s, it has not been reported that it can be expressed on a large scale in a eukaryotic expression system. Purification of the protein may be caused by the unstable structure of the G protein expressed alone. In order to solve this important technical problem, the present invention introduces a zipper peptide that is easy to form a trimer at the carboxyl end of the G protein to ensure the stability of the G protein. The structure is correctly folded...
Embodiment 2
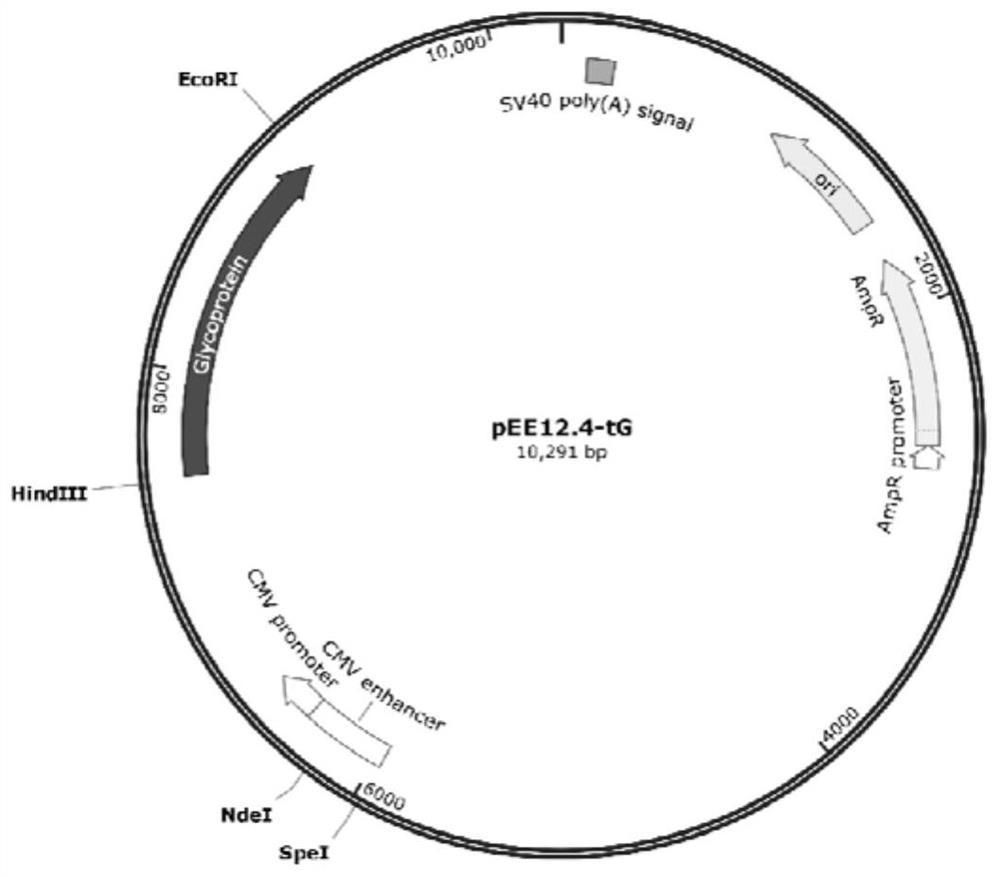
[0049] Example 2: Construction of pEE12.4-OPTI-tG recombinant plasmid
[0050] 2.1 PCR amplification of target fragment OPTI-tG
[0051] 2.1.1 PCR reaction
[0052] (1) Primer design and synthesis
[0053] Upstream primer: 5'-ACGAAGCTTGCCGCCACCATGGTGCCTCAGGTGC-3'
[0054] Downstream primer: 5'-GCGAATTCTTAATGGTGATGGTGATGGTGTGTGCGATTGC-3'
[0055] (2) Add 50 μL of the sample system, as shown in the table below:
[0056]
[0057] PCR amplification program:
[0058]
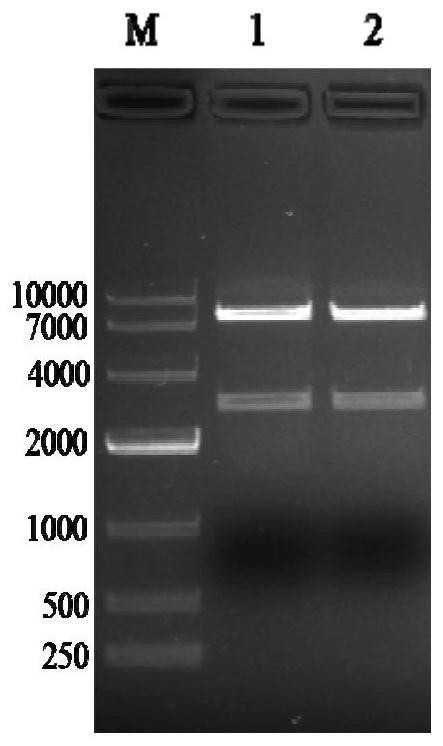
[0059] 2.1.2 Gel recovery of PCR products
[0060] (1) Mark the sample collection EP tube, adsorption column and collection tube;
[0061] (2) Take the weight of the marked empty EP tube, and record the value;
[0062] (3) Carefully cut out a single target DNA band from the agarose gel with a scalpel on a gel cutter and put it into a clean 1.5mL centrifuge tube;
[0063] (4) Add 600 μL PC buffer to the 1.5mL centrifuge tube in step (3), place in a 50°C water bath for about 5 minutes, and gently turn the...
Embodiment 3
[0110] Example 3: Establishment of transfection of pEE12.4-OPTI-tG recombinant plasmid into CHO-K1 cells and monoclonal screening
[0111] 3.1 CHO-K1 cell transfection
[0112] (1) Preparation: UV sterilization in a biological safety cabinet for 30 minutes; DMEM / F12 (containing 10% serum, 1% double antibody), DMEM / F12 and PBS were placed in a 37°C water bath and preheated to 37°C.
[0113] (2) Take out the cells (10 cm cell culture dish) from the incubator at 37° C., discard the supernatant medium, wash the cells once with pre-warmed 8 mL PBS, and discard the PBS.
[0114] (3) Add 1-2mL 0.25% trypsin-EDTA to each 10cm cell culture dish, digest at room temperature for about 2 minutes, observe under the microscope that the cells shrink and become round, and appear as single cells.
[0115] (4) Add 4 mL of DMEM / F12 (containing 10% serum, 1% double antibody) to terminate the digestion reaction, and blow the cells away with a pipette.
[0116] (5) Transfer the digested cells to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com