A method for synthesizing deuterated methylamine hydrochloride and deuterated dimethylamine hydrochloride with boc-protected benzylamine
A technology of deuterated dimethylamine hydrochloride and deuterated methylamine hydrochloride, which is applied in the field of organic synthesis and achieves the effects of serious environmental pollution, few by-products and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
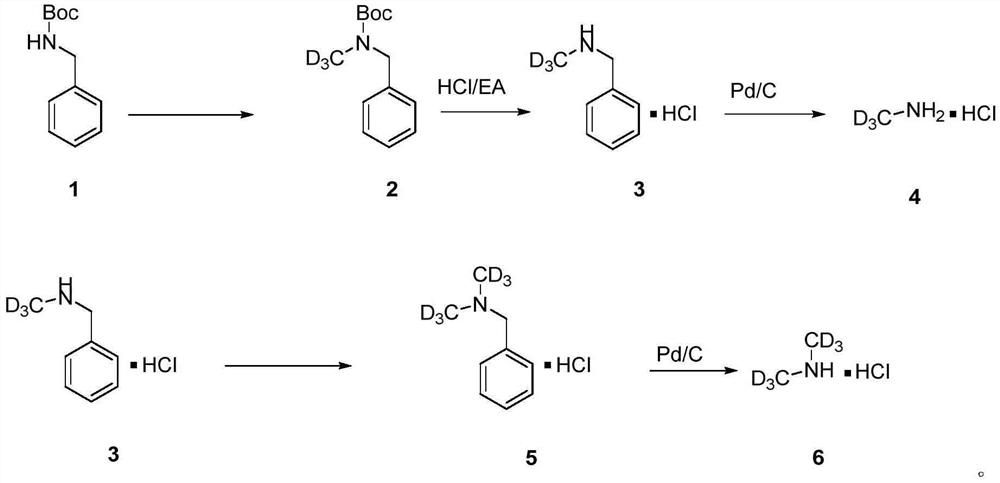
[0039] Preparation of deuterated methylamine hydrochloride
[0040]
[0041] Synthesis of Compound 2
[0042] Boc-protected benzylamine (24.12 mmol) (CAS42116-44-9) was added to 25 ml DMF at 0°C under nitrogen protection. Then NaH (60%, 26.54mmol) was added dropwise, and after stirring for 30min, TsOCD dissolved in 5mL DMF was added dropwise 3 (24.12 mmol) (CAS 7575-93-1), then the reaction mixture was heated to room temperature, and after the completion of the reaction was confirmed by thin-layer chromatography analysis, saturated ammonium chloride (40 mL) was added at room temperature to quench the reaction. Extracted with EA (ethyl acetate) and water (3×50ml), combined the organic layers in anhydrous Na 2 SO 4 dried, filtered and concentrated by rotary evaporation to obtain the crude product. Compound 2 (23.15 mmol) was obtained through silica gel column chromatography (EA:PE=1:10) with a yield of 96%; 1 H NMR (400MHz, CDCl 3 ):δ1.47(s,9H,-CH 3 ),4.41(s,2H,-CH 2 ...
Embodiment 2
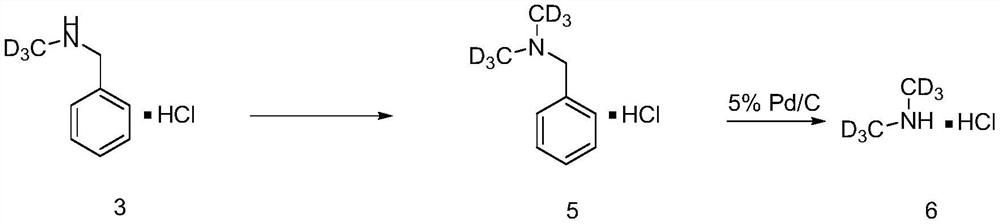
[0048] Preparation of deuterated dimethylamine hydrochloride
[0049]
[0050] Synthesis of compound 5
[0051] React as in Example 1 to obtain compound 3N-benzyldeuteromethylamine hydrochloride. Add N-benzyldeuteromethylamine hydrochloride (16.10mmol) to 16mL of anhydrous THF under nitrogen protection at -20°C, then add n-butyllithium (16.10mmol) dropwise, stir for 30min, then add 20mL dropwise TsOCD 3 (16.10 mmol) of anhydrous THF solution was reacted for 30 minutes, the reaction mixture was warmed to room temperature and reacted overnight. After completion of the reaction as determined by thin layer chromatography analysis, the reaction was quenched with saturated ammonium chloride (20 mL) at room temperature. Extracted with water and EA (3×30ml), combined organic layers, anhydrous Na 2 SO 4 The crude product was obtained by drying, filtering and rotary evaporation. Silica gel column chromatography (DCM:MeOH20:1) obtained compound 5 (14.68mmol), yield 91%; 1 H NMR...
Embodiment 3
[0063] Embodiment 3 is identical with embodiment 1 preparation method, and difference is:
[0064] Compound 2 was synthesized at -20°C using potassium tert-butoxide and deuteromethyl iodide, and the molar ratio of compound 1 to deuteromethyl iodide was 4:1;
[0065] In the synthesis of compound 3, the reaction molar ratio of compound 2 and HCl is 1:1, and the solvent dimethyl sulfoxide of the HCl solution;
[0066] In the preparation of deuterated methylamine hydrochloride, palladium carbon (0.5% palladium), the reaction temperature is 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com