CuPc-at-Ti3C2TxMXene catalytic material, electrode and application of CuPc-at-Ti3C2TxMXene catalytic material and electrode in nitrate radical reduction
A catalytic material and electrode technology, applied in the application field of nitrate reduction to produce ammonia, can solve the problems of low conversion rate, poor selectivity to ammonia, by-products, and high nitrite concentration, and achieve low by-products and easy-to-obtain raw materials. , easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
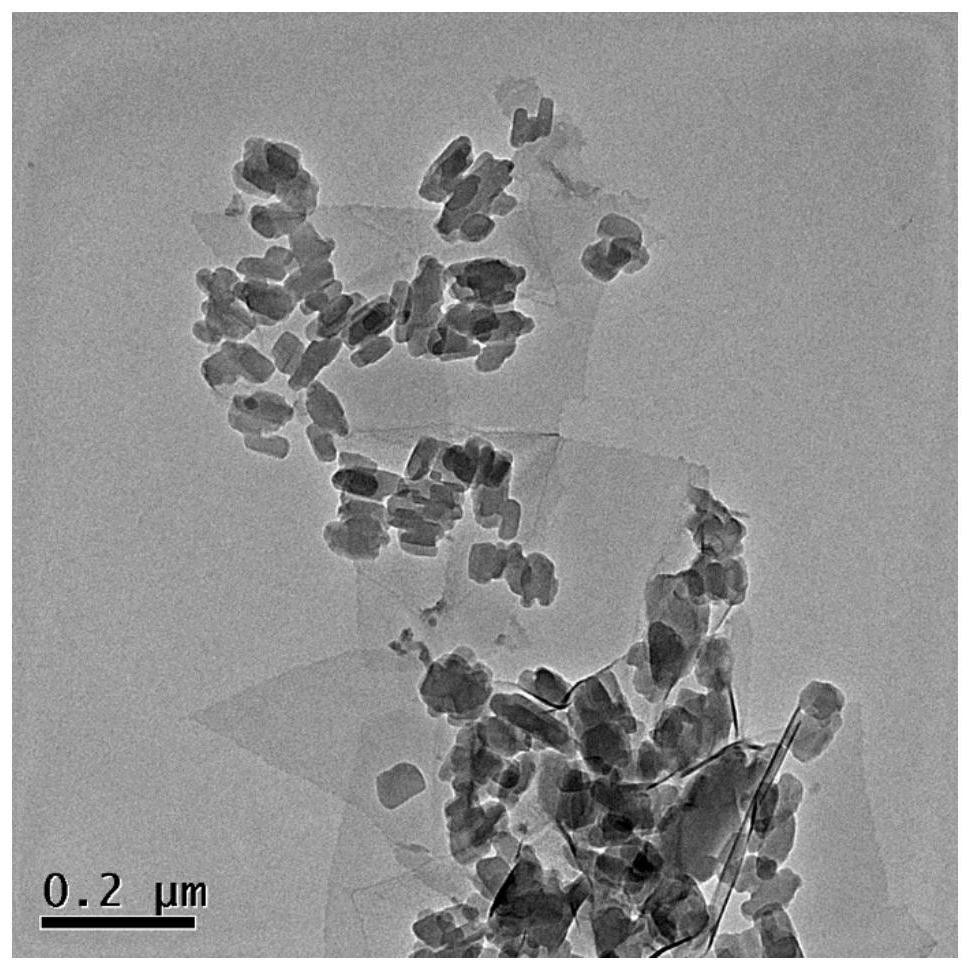
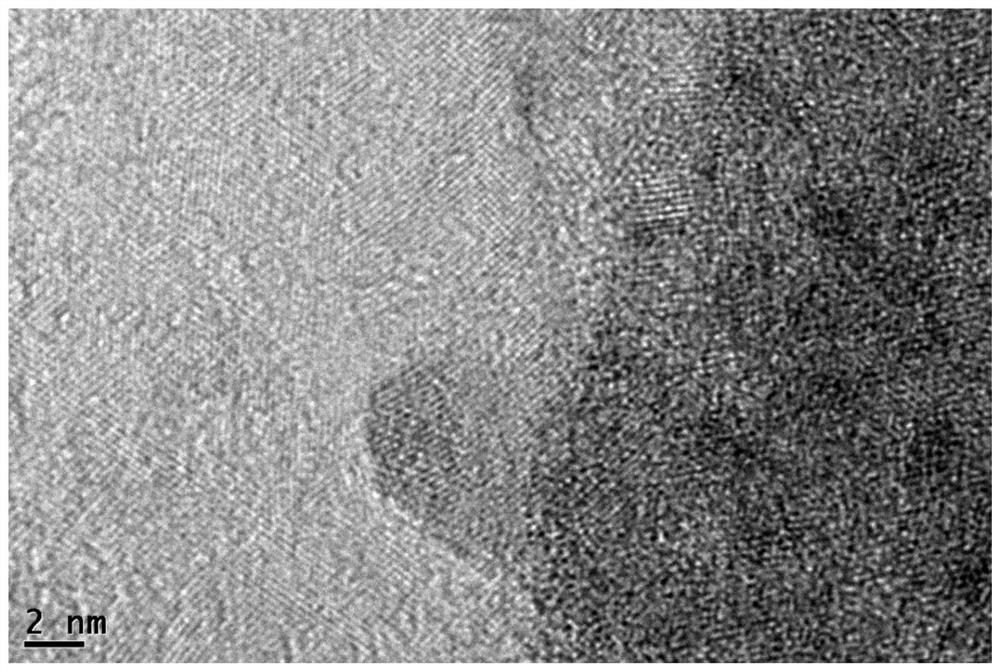
[0028] CuPc@Ti 3 C 2 T x The synthesis of MXene catalytic materials, the specific steps are as follows:
[0029] At room temperature, measure 5mg / ml of Ti 3 C 2 T x MXene (purchased from Shandong Xene Research New Material Technology Co., Ltd.) 1mL in a flask, and then add 49 mL of anhydrous methanol to obtain Ti 3 C 2 T x MXene solution.
[0030] According to the ratio of the amount of different substances of copper and titanium (the ratio of Cu to Ti is 10%, 20%, 30%, and 50% respectively), add 100mL copper phthalocyanine ethanol solution dropwise to the The above Ti 3 C 2 T x In the MXene solution, the addition rate was 1mL / min. After the addition was completed, it was centrifuged at a centrifugal speed of 7800 rpm for 10 minutes, washed with absolute ethanol, and centrifuged and washed 3 times in total. Finally, the supernatant after centrifugation was poured out to obtain the The precipitate was vacuum-dried at 60°C for 3 hours, and it was CuPc@Ti 3 C 2 ...
Embodiment 2
[0033] The preparation of an electrode for electrocatalytic reduction of nitrate to produce ammonia, the specific steps are as follows:
[0034] Weigh the vacuum-dried CuPc@Ti 3 C 2 T x Mix MXene catalytic material with 100 microliters of absolute ethanol and 100 microliters of Nafion binder solution (acting as a binder, a commercially available product) to obtain a catalyst slurry; then use a pipette gun to draw 100 microliters of catalyst slurry for drop coating On a carbon cloth hydrophilic surface treated with oxygen plasma; the carbon cloth after drop coating was dried at room temperature for 3 h, thus preparing CuPc@Ti 3 C 2 T x MXene composite electrode device, an electrode for electrocatalytic reduction of nitrate to produce ammonia, CuPc@Ti on carbon cloth 3 C 2 T x The loading capacity of MXene catalytic material is 2.7mg / cm 2 .
Embodiment 3
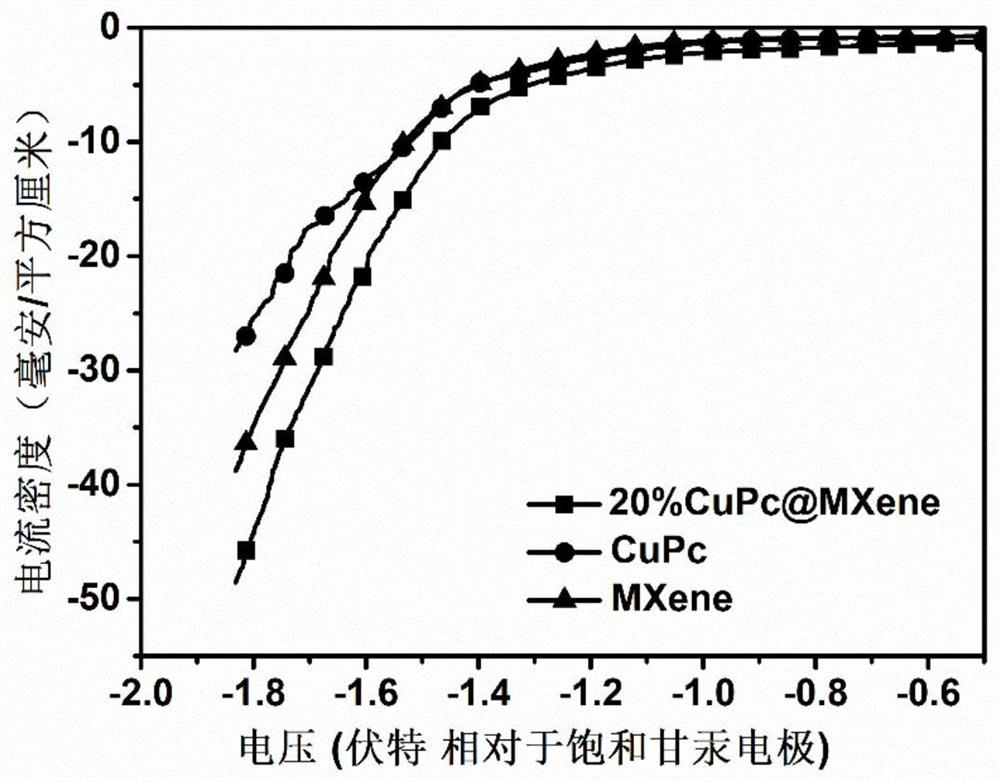
[0036] CuPc@Ti 3 C 2 T x MXene catalytic materials were tested by an electrochemical workstation (model CorrTest CS310). Before the test, the working electrode was connected to the carbon cloth of the electrode for electrocatalytic reduction of nitrate to produce ammonia, the platinum sheet was used as the counter electrode, the calomel electrode was used as the reference electrode, and the electrolytic cell was an H-type electrolytic cell. After the assembly is completed, 0.5mol / L sodium sulfate is used as the electrolyte, and nitrate with a nitrogen concentration of 30mg / L~200mg / L is used as the electrolyte. The main electrochemical test is linear voltammetry scanning method, and the scanning potential range is -0.1~ -1.85V, constant voltage method, applied voltage range -1.45~-1.85V.
[0037] attached image 3 It is the linear sweep voltammetry curve of CuPc, MXene and 20% CuPc@MXene catalytic materials. It can be seen from the figure that the composite material shows ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com