A method for preparing hydrophilic phytosterol dibasic acid sugar esters in an organic phase by enzymatic method
A technology of dibasic acid sugar ester and phytosterol, which is applied in the field of preparing hydrophilic phytosterol dibasic acid sugar ester by whole enzymatic method, can solve the problems of low food safety factor, large amount of catalyst used, complicated post-processing, etc. Good public acceptance, improved hydrophilicity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of β-Sitosterol Vinyl Adipate
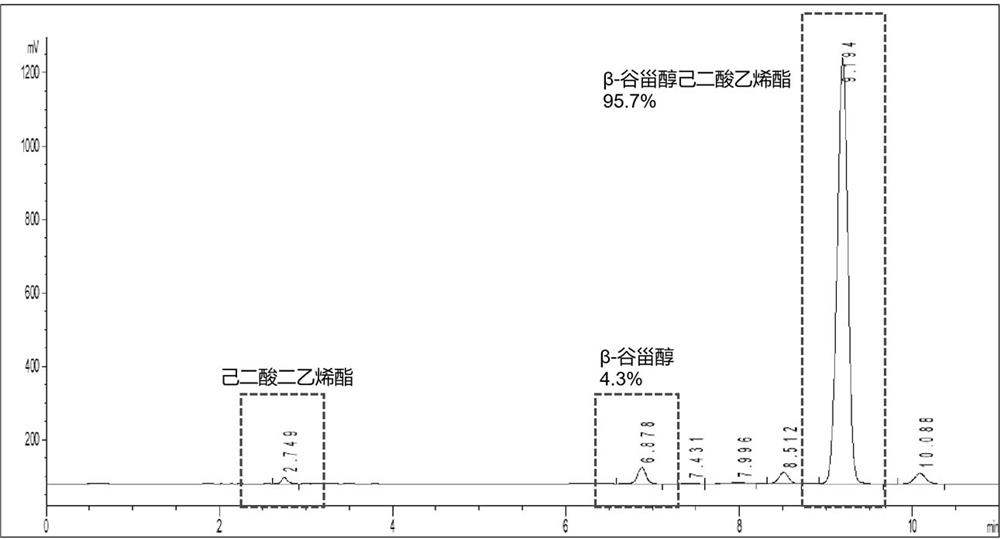
[0042] Preparation method: respectively add 3 g β-sitosterol, 3 ml divinyl adipate, 0.5 g Candida rugosa lipase Candida Rugosa, and 100 ml dehydrated isooctane into the reaction flask in turn, and place in a constant temperature shaking reactor , start the shaking speed at 250 rpm, adjust the temperature to 50 °C, and react for 14 h. The conversion rate of β-sitosterol vinyl adipate was detected by HPLC-ELSD up to 95.7%. figure 1 High performance liquid chromatogram for β-sitosterol vinyl adipate. The reaction solution was filtered to remove the enzyme, and the solvent was removed by rotary evaporation to obtain 3.7 g of β-sitosterol vinyl adipate.
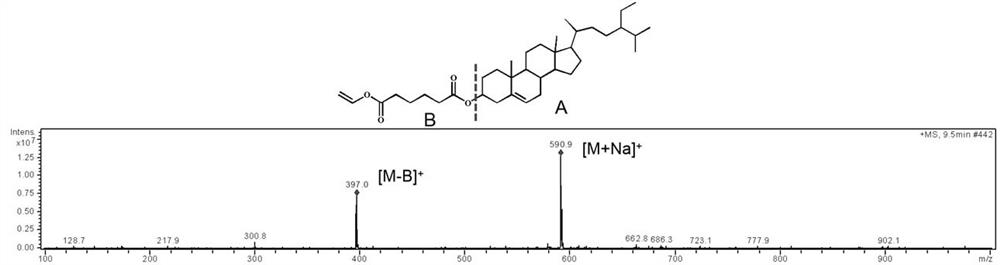
[0043] Structural identification: HPLC-MS: The relative molecular mass of β-sitosterol is 414 respectively. The relative molecular mass of β-sitosterol vinyl adipate is 568. β-sitosterol vinyl adipate in ES + Under ionization, [M+Na] in the presence of β-sitosterol vinyl adip...
Embodiment 2
[0045] Preparation of campesterol sucrose adipate
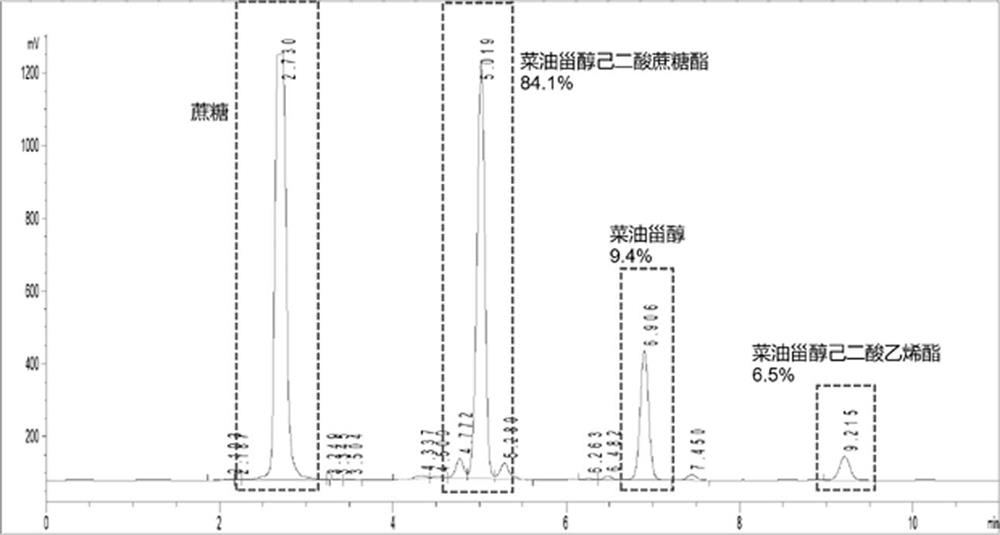
[0046] Preparation method: (1) respectively add 3 g campesterol, 3 ml divinyl adipate, 1 g Novozymes immobilized lipase Novozyme 435, and 100 ml dehydrated petroleum ether into the reaction bottle in sequence, and place at constant temperature to shake In the reactor, start the shaking speed at 250 rpm, adjust the temperature to 55 °C, and react for 48 h. The conversion rate of campesterol vinyl adipate can reach 81.7% as detected by HPLC-ELSD. (2) After sampling, filter the reaction liquid in the first step to remove the enzyme, remove the solvent by rotary evaporation, add 6 g of hydrophilic modifier sucrose, 0.2 g of alkaline protease, and 30 ml of pyridine, place the reaction bottle in a constant temperature shaking reactor, and open The shaking speed was 250 rpm, the temperature was adjusted to 55 °C, and the reaction was carried out for 96 h. Sampling is carried out for HPLC-ELSD analysis, and the conversion rate of t...
Embodiment 3
[0049] Preparation of β-Sitosterol Succinate Sorbitan Ester
[0050] Preparation method: (1) Add 3 g of β-sitosterol, 3 ml of divinyl succinate, 1 g of immobilized lipase Lipozyme RM, and 80 ml of dehydrated n-heptane into the reaction bottle in sequence, and place it in a constant temperature shaking reaction Turn on the shaker at 250 rpm, adjust the temperature to 60 °C, and react for 48 h. The conversion rate of phytosterol vinyl adipate can reach 97.1% as detected by HPLC-ELSD. (2) After sampling, filter the reaction solution in the first step to remove the enzyme, remove the solvent by rotary evaporation, add 3 g of hydrophilic modifier sorbitol, 0.5 g of neutral protease, and 20 ml of binary mixed solvent of pyridine and acetone, and the reaction The bottle was placed in a constant temperature shaking reactor, the shaking speed was turned on at 250 rpm, the temperature was adjusted to 40 °C, and the reaction was carried out for 120 h. Sampling is carried out for HPLC-E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com