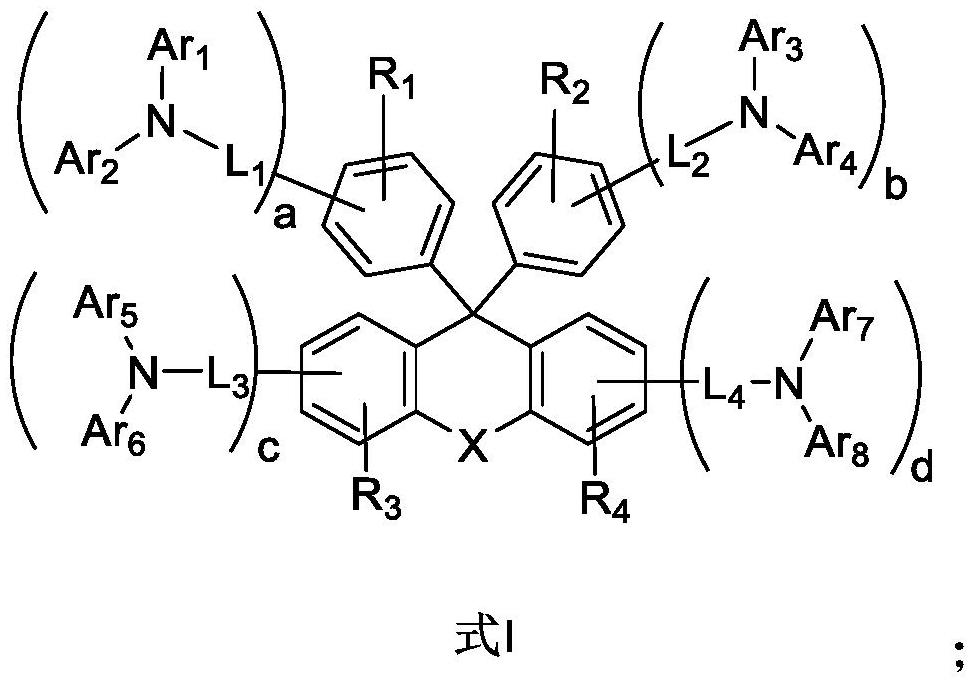
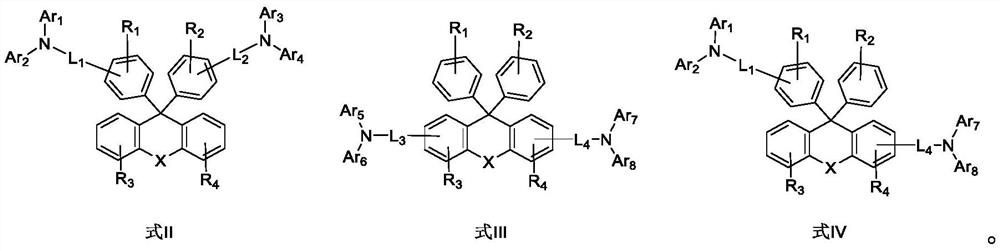
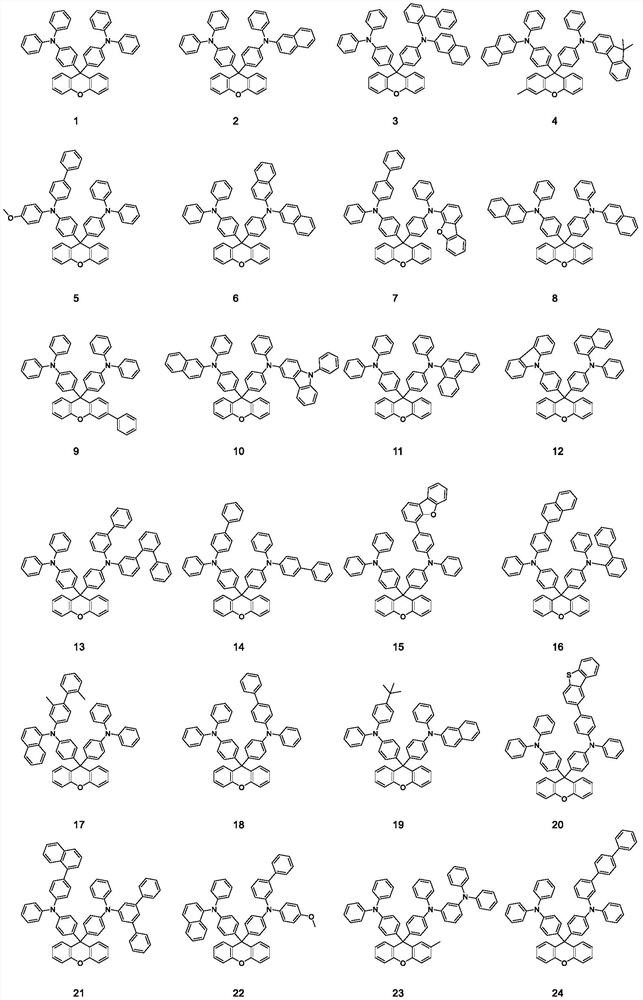
Luminescent material based on fluorene arylamine compounds, preparation method of luminescent material and organic electroluminescent device
A technology of luminescent materials and compounds, applied in luminescent materials, electrical solid devices, silicon organic compounds, etc., can solve the problems of destroying hole-electron charge balance, quantum efficiency and service life deterioration, and quantum efficiency reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] This material example provides a luminescent material based on fluorene aromatic amine compounds, and its synthesis route is as follows:
[0085]
[0086] Its preparation method is as follows:
[0087] (1) Add reactant B-4 (110mmol) into a three-necked flask, add 500mL of anhydrous tetrahydrofuran, replace with nitrogen three times, then cool the reaction system to -78°C, and add (2.5M) n-BuLi (110mmoL) dropwise , stirred at -78°C for 2h. Reactant A-4 (100mol) was dissolved in tetrahydrofuran and added dropwise to the reaction system. After the dropwise addition was completed, the temperature was raised to room temperature and stirred for 10 h. Distilled water was added to terminate the reaction, the organic phase was collected by liquid separation, and dried by adding anhydrous magnesium sulfate. The remaining water was removed, anhydrous magnesium sulfate was removed by filtration, and the organic phase was removed by a rotary evaporator to obtain a solid organic...
Embodiment 2
[0097] This material example provides a luminescent material based on fluorene aromatic amine compounds, and its synthesis route is as follows:
[0098]
[0099] Its preparation method is as follows:
[0100] (1) Add reactant B-50 (110mmol) into a three-necked flask, add 500mL of anhydrous tetrahydrofuran, replace with nitrogen three times, then cool the reaction system to -78°C, and add (2.5M) n-BuLi (110mmoL) dropwise , stirred at -78°C for 2h. The reactant A-50 (100mol) was dissolved in tetrahydrofuran and added dropwise to the reaction system. After the dropwise addition was completed, the temperature was raised to room temperature and stirred for 10 h. Distilled water was added to terminate the reaction, the organic phase was collected by liquid separation, and dried by adding anhydrous magnesium sulfate. The remaining water was removed, anhydrous magnesium sulfate was removed by filtration, and the organic phase was removed by a rotary evaporator to obtain a solid o...
Embodiment 3
[0110] This material example provides a luminescent material based on fluorene aromatic amine compounds, and its synthesis route is as follows:
[0111]
[0112] Its preparation method is as follows:
[0113] (1) Add reactant B-75 (110mmol) into a three-necked flask, add 500mL of anhydrous tetrahydrofuran, replace with nitrogen three times, then cool the reaction system to -78°C, and add (2.5M) n-BuLi (110mmoL) dropwise , stirred at -78°C for 2h. The reactant A-75 (100mol) was dissolved in tetrahydrofuran and added dropwise to the reaction system. After the dropwise addition was completed, the temperature was raised to room temperature and stirred for 10 h. Distilled water was added to terminate the reaction, the organic phase was collected by liquid separation, and dried by adding anhydrous magnesium sulfate. The remaining water was removed, anhydrous magnesium sulfate was removed by filtration, and the organic phase was removed by a rotary evaporator to obtain a solid o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com