Preparation of 4-bromo-2-(4 '-ethoxy-benzyl)-1-chlorobenzene
An ethoxy and benzyl technology, applied in the field of compound synthesis, can solve the problems of short steps, good selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
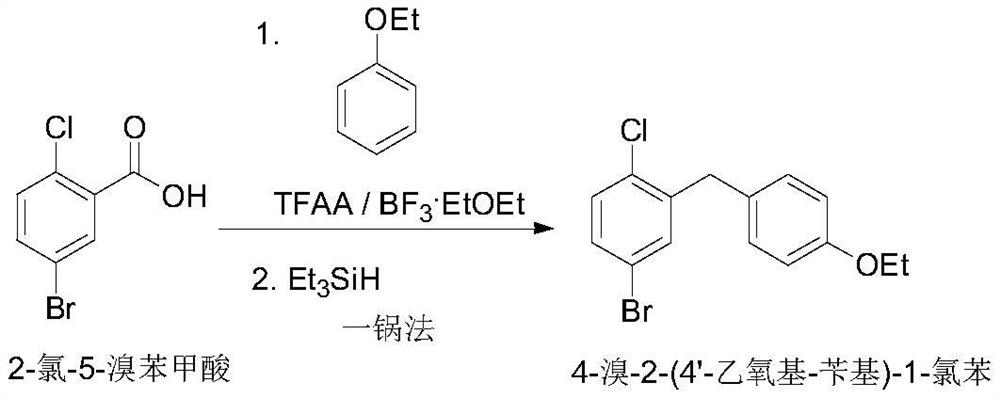
[0018] Embodiment one: the synthesis of 4-bromo-2-(4'-ethoxy-benzyl)-1-chlorobenzene
[0019] Add trifluoroacetic anhydride (84.0g, 400mmol), phenetole (14.7g, 120mmol), boron trifluoride etherate (1.42g, 10mmol) successively into a 250mL four-necked bottle, and add 2-chloro-5- Bromo-benzoic acid (23.5g, 100mmol) was heated to 30±5°C with stirring for 6 hours to obtain a dark brown solution. After cooling down to room temperature, triethylsilane (34.9 g, 300 mmol) was added, and the temperature was raised to 55-60° C. for 18 hours. After cooling down, concentrate under reduced pressure to remove the low-boiling point solvent. Add dichloromethane (150 mL) and saturated sodium bicarbonate solution (60 mL) to the residue to wash and separate the layers. The collected organic layer is washed twice with water (60 mL×2) and then concentrated. The residue was recrystallized by adding ethanol, filtered, and air-dried at 40°C to obtain the white title compound (24.3 g, yield 74.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com