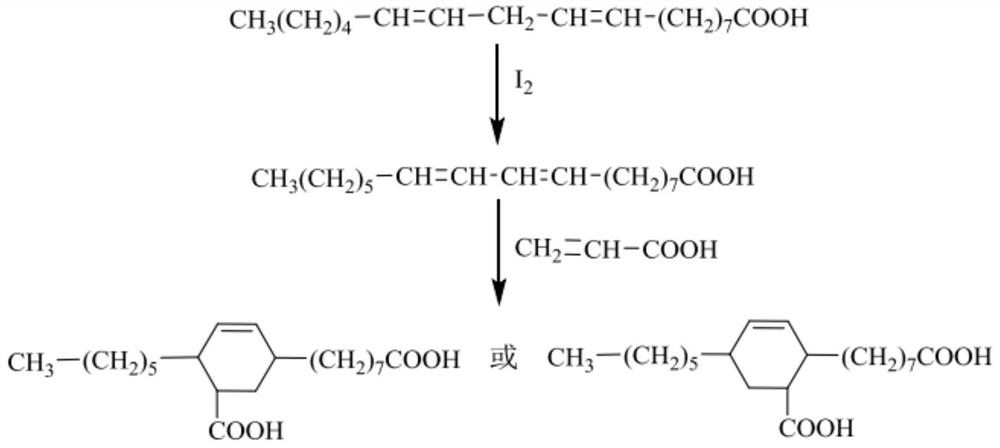
Method for preparing C21 binary acid by using micro-channel technology
A dibasic acid and microchannel technology, which is applied in the preparation of carboxylate, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of increased personnel input, increased production costs, and increased temperature, and achieve thermal quality The effect of high transfer rate, high production efficiency and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
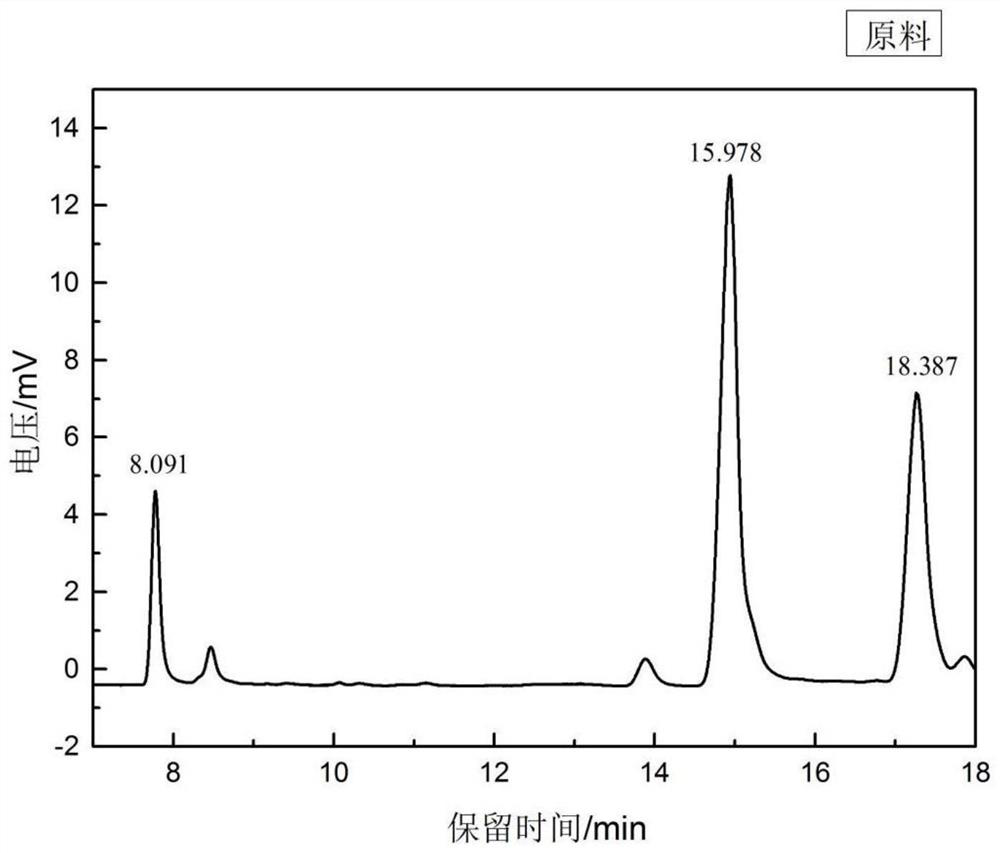
[0042] (1) Get raw material oleic acid to carry out methyl esterification reaction, carry out quantitative analysis with GC, adopt area normalization method to calculate and obtain each component content is palmitic acid: oleic acid: linoleic acid=10.1943%: 56.9693%: 32.8364% .
[0043] (2) Put 150 g of oleic acid and 0.098 g of catalyst iodine into an autoclave, replace the air in the autoclave with nitrogen, stir magnetically and heat up to 150° C. for 2 h to obtain an intermediate product.
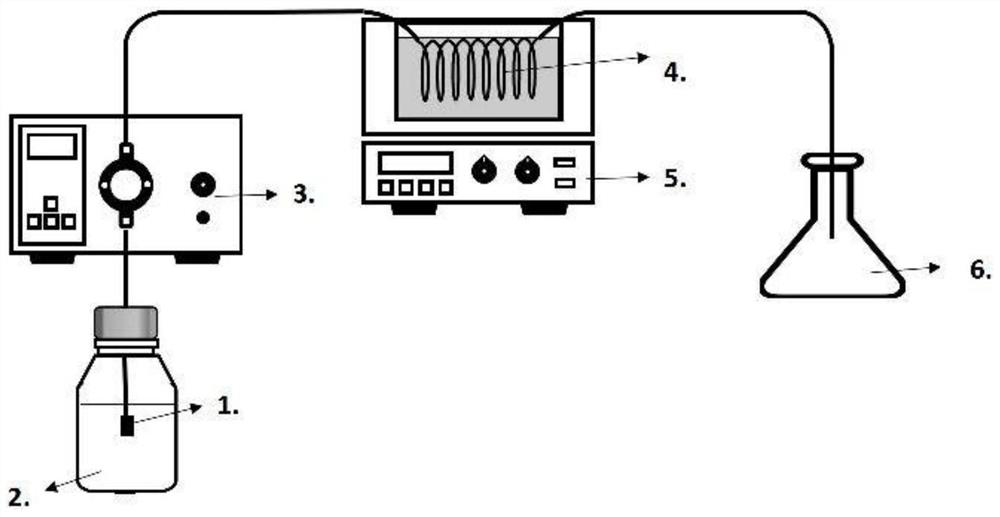
[0044](3) Connect the reaction device as required, place the pipeline reactor in an oil bath and heat it to 180 degrees.
[0045] (4) Add acrylic acid (n (acrylic acid): n (linoleic acid) = 1.2: 1) to the intermediate product obtained, add acrylic acid quality 1% inhibitor hydroquinone as reactant, under heating condition Stay for 3h, pressurize at 100psi, and collect the reaction product with an Erlenmeyer flask.
[0046] (5) Carry out methyl esterification reaction with the reaction...
Embodiment 2
[0049] Treatment process, treatment time, and pressure are the same as in Example 1, except that the reaction temperature of the oil bath is 170 degrees. The reaction product was taken for methyl esterification reaction, quantitatively analyzed by GC, and the content of each component was calculated by the area normalization method, and the calculated conversion rate was 89%. Spectrum such as Figure 5 Shown, retention time 17.112min represents the peak of linoleic acid, and image 3 The peak area of linoleic acid in the gas phase diagram increases significantly, indicating that the reaction temperature in the pipeline reactor increases and the conversion rate increases. This is because the temperature increases, the effective collision frequency of reactant molecules increases, and the reaction rate accelerates.
Embodiment 3
[0051] The treatment process, treatment time and treatment temperature are the same as in Example 1, except that the reaction pressure in the pipeline reactor is 40 psi. The methyl esterification reaction was carried out on the reaction product, quantitative analysis was carried out by GC, and the content of each component was calculated by the area normalization method, and the calculated conversion rate was 68%. Spectrum such as Figure 6 Shown, retention time 17.145min represents the peak of linoleic acid, and Figure 4 The peak area of oleic acid in the gas phase diagram increases significantly, indicating that the reaction pressure increases and the conversion rate increases.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com