A fused ring quinoxaline imide-based non-fullerene acceptor material and its preparation method and application
A quinoxaline imide-based, non-fullerene acceptor technology, which is applied in the field of fused ring quinoxaline imide-based non-fullerene acceptor materials and its preparation, and can solve the lack of matching types of donor materials , shallow HOMO energy level, low open circuit voltage, etc., to achieve the effect of improving carrier mobility, increasing interaction, and enhancing π-π interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
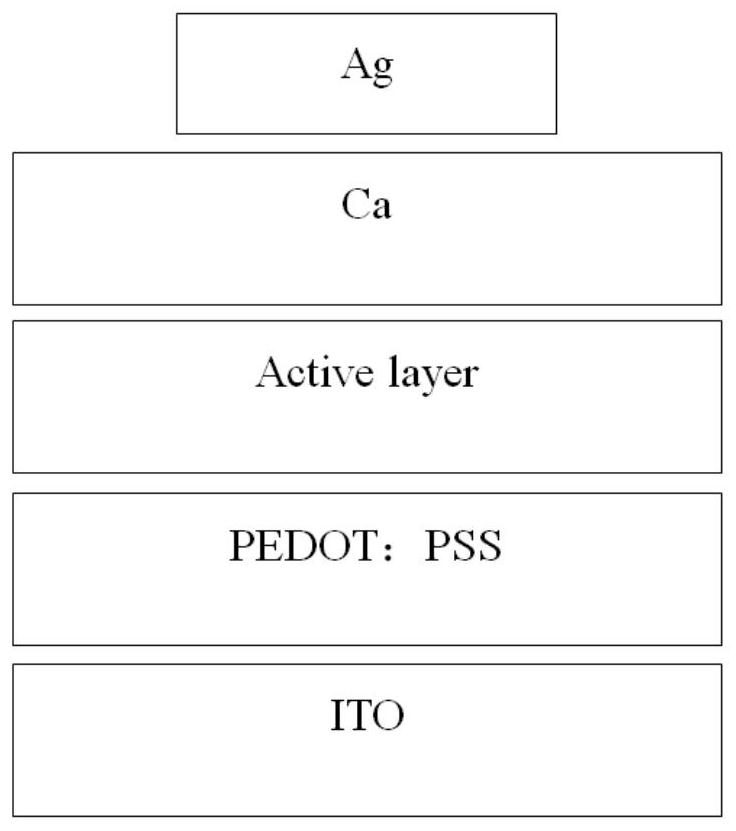
Image
Examples
specific Embodiment 1
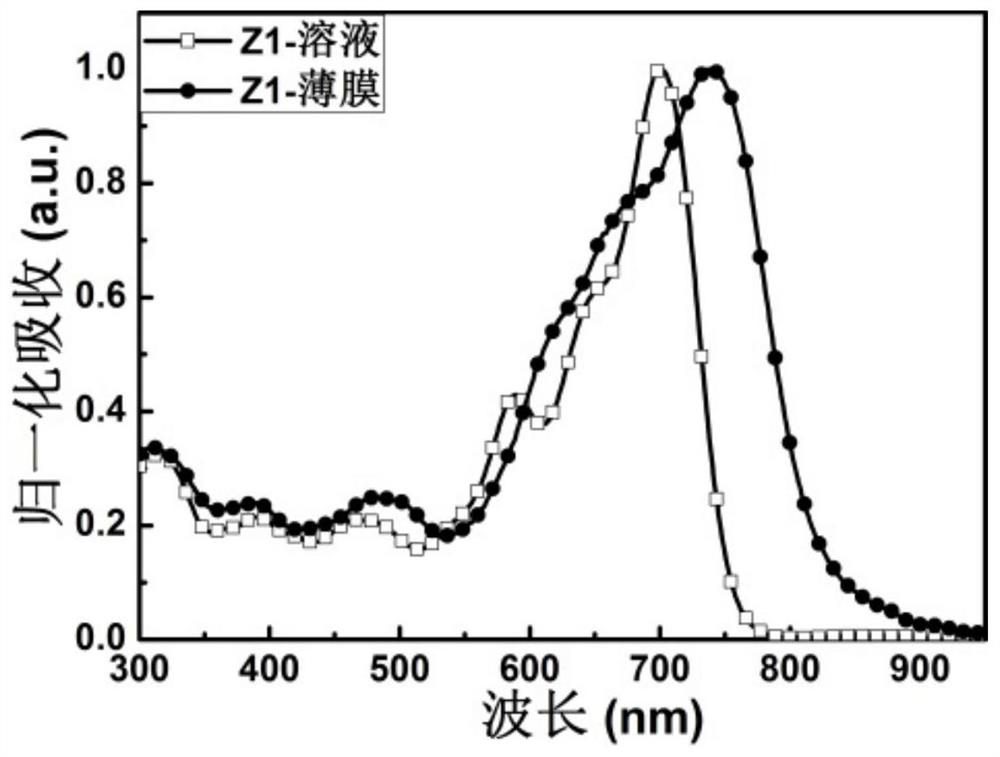
[0094] A fused ring quinoxaline imide-based non-fullerene acceptor material whose chemical structure is Z1, its synthetic route is as follows:
[0095]
[0096] (1) Synthesis of an intermediate whose chemical structure is b: under nitrogen protection, add 12,13-bis(2-ethylhexyl)-3,9-dodecane-12,13-dihydro- [1,2,5]thiadiazolo[3,4-e]thiophene[2",3":4',5']thiophene[2',3':4,5]pyrrole[3,2- g] Thiophene[2',3':4,5]thiophene[3,2-b]indole (a) (0.001mol), iron powder (0.01mol), 0.07L acetic acid solvent. After refluxing for 10 hours, cool to room temperature. Using dichloromethane for extraction, the organic phase was dried with magnesium sulfate, and the solvent was spin-dried to obtain a crude product. The crude product was directly used in the next step without purification.
[0097] MALDI-TOF-MS: m / z=943.57 (M + ).
[0098] (2) Synthesis of an intermediate with the chemical structural formula d: under nitrogen protection, compound b (0.001 mol), diethyl dioxosuccinate (0.01 mo...
specific Embodiment 2
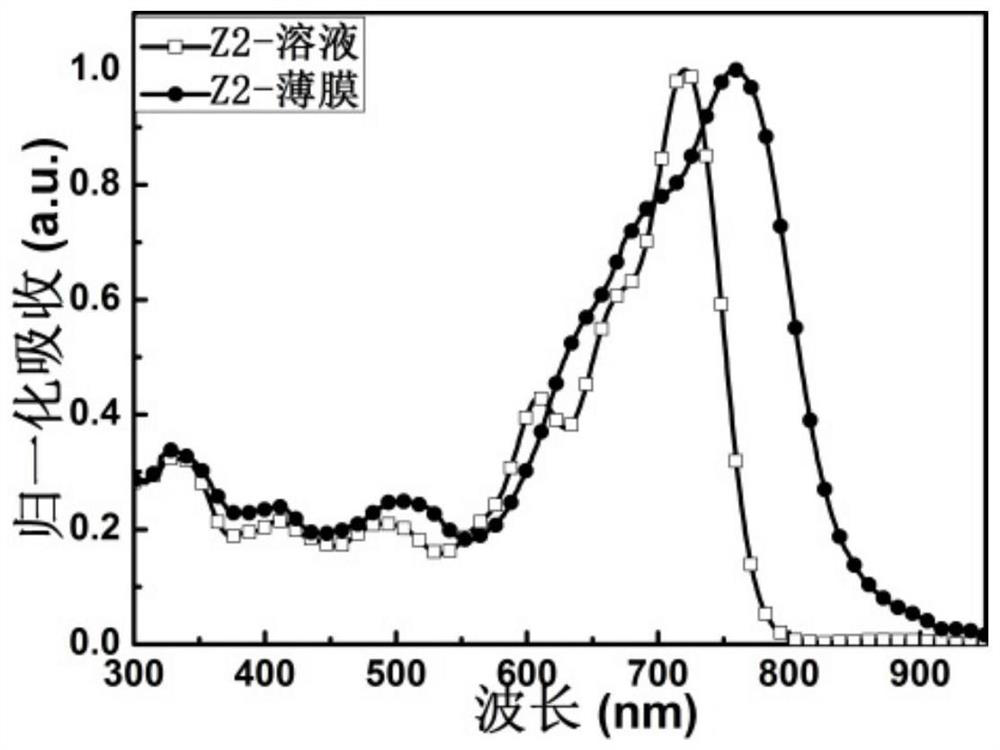
[0123] A kind of condensed ring quinoxaline imide based non-fullerene acceptor material whose chemical structure is Z2, its synthetic route is as follows:
[0124]
[0125] The experimental procedure of the fused ring quinoxaline imide-based non-fullerene acceptor material Z2 is basically the same as that of Example 1, and compound h is prepared according to the experimental procedure of Example 1; compound h and 5,6-dichloro-3- (Dicyanomethylene) indoketone (2ClINCN) reacted to obtain fused ring quinoxaline imide-based non-fullerene acceptor material Z2.
[0126] Synthesis of fused ring quinoxaline imide-based non-fullerene acceptor material with chemical structural formula Z2: under nitrogen protection, compound h (0.001mol), 5,6-dichloro-3-( Dicyanomethylene) indoketone (2ClINCN) (0.003 mol), pyridine (0.001 mol) and 0.04 L of chloroform solvent. After refluxing for 12 hours, cool to room temperature. Extract with dichloromethane, dry the organic phase with anhydrous m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com