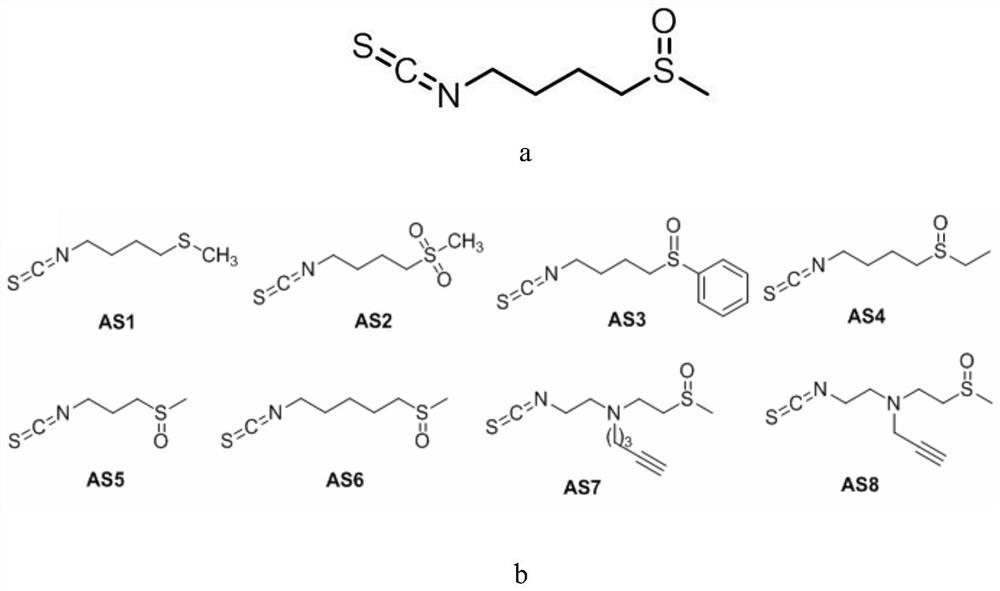
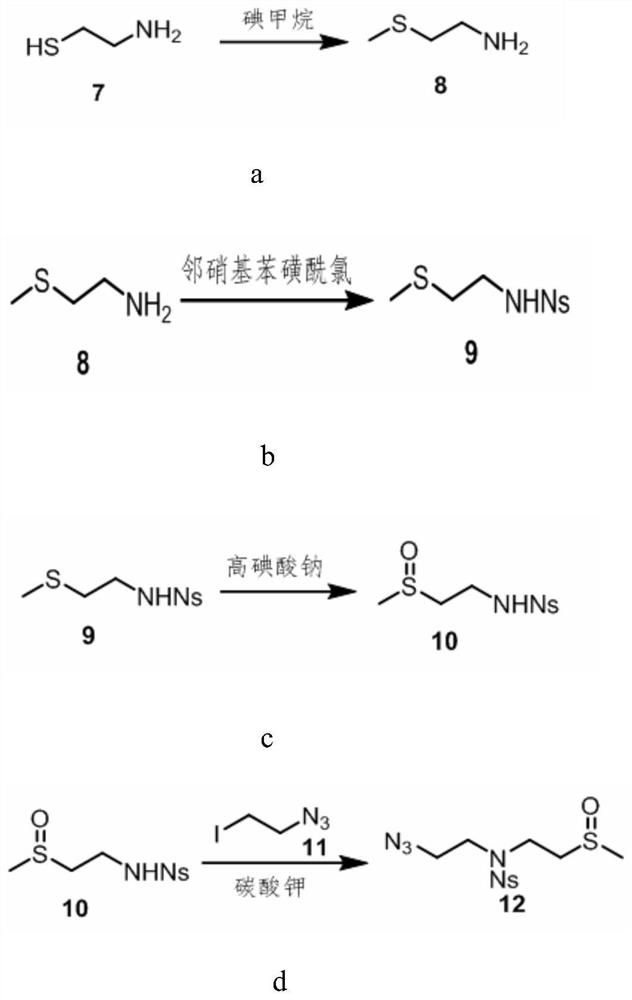
Application of sulforaphane and derivatives thereof as bacterial effector protein transcription inhibitor
A technology of sulforaphane and effector protein, applied in the biological field, can solve problems such as drug resistance and destruction of beneficial microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
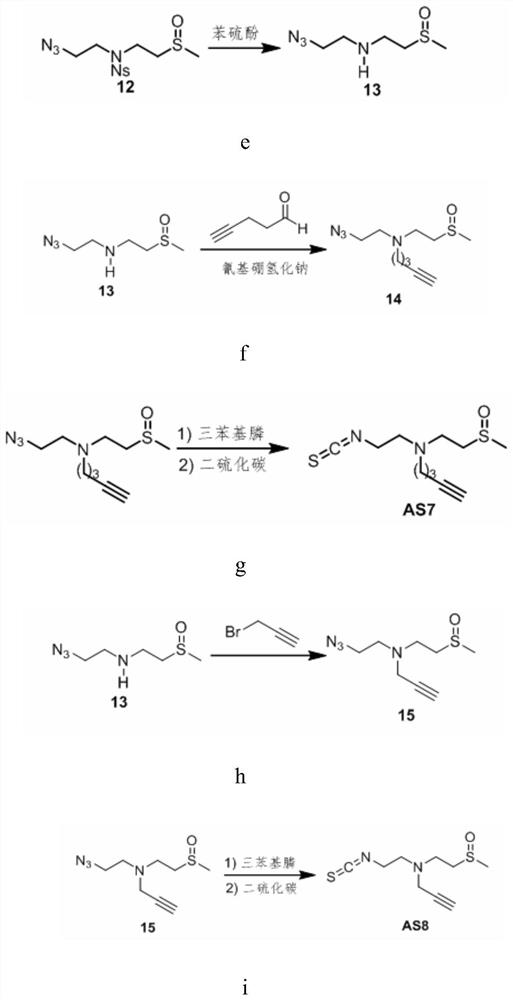
[0073] Embodiment 1, sulforaphane derivative AS7 and its synthesis method
[0074] Follow the steps below to synthesize AS7:
[0075] (1) Synthesis of Compound 8:
[0076]
[0077] Mercaptoethylamine (compound 7) (300mg, 3.88mmol, 1eq) (purchased from Tissier, catalog number: 60-23-1) and sodium hydroxide (233mg, 5.82mmol, 1.5eq) were dissolved in ethanol ( 7.5 mL), iodomethane (290 μL, 4.66 mmol, 1.2 eq) was added at 0°C. The mixture was stirred at room temperature for 3 hours, then spin-dried and redissolved in chloroform and filtered to obtain the crude product of compound 8 ( figure 2 A).
[0078] (2) Synthesis of Compound 9:
[0079]
[0080] The crude compound 8 was dissolved in ethyl acetate (30 mL) and saturated sodium bicarbonate (20 mL), and o-nitrobenzenesulfonyl chloride (1.29 g, 5.82 mmol, 1.5 eq) was added at 0°C. The mixture was stirred at room temperature for 3 hours, the organic phase was washed with water, concentrated by rotary evaporation to obt...
Embodiment 2
[0097] Embodiment 2, sulforaphane derivative AS8 and its synthesis
[0098] Follow the steps below to synthesize AS8:
[0099] (1) Synthesis of Compound 15:
[0100]
[0101] Compound 13 (40 mg, 0.22 mmol) was dissolved in 2 mL of ether, 3-bromopropyne and cesium carbonate were added at 0°C, and then stirred at room temperature for 15 hours. Quenched with 5 mL of water, extracted with dichloromethane (10 mL×3), dried over anhydrous sodium sulfate, filtered to remove the desiccant, and evaporated the solvent under reduced pressure. Purification by column chromatography (dichloromethane / methanol=50:1) gave compound 15 (20 mg, 43%) as a colorless liquid ( figure 2 h).
[0102] (2) Synthesis of compound AS8:
[0103]
[0104] Compound 15 (10.7 mg, 0.05 mmol) was dissolved in 2 mL of diethyl ether, triphenylphosphine was added, and stirred at 40° C. for 4 hours. Cool to room temperature, add 2 mL of carbon disulfide, and reflux for 5 hours. The solvent was evaporated u...
Embodiment 3
[0106] Example 3, the application of sulforaphane in inhibiting the expression of Pseudomonas syringae effector protein-related genes
[0107] 1. Sulforaphane inhibits the expression of effector proteins in Pseudomonas syringae
[0108] 1. Pick a single colony of Pst DC3000ΔsaxAB / F / D / G and place it in KB medium, culture overnight at 28°C and 220rpm, centrifuge at 4000rpm for 10 minutes, and collect the bacteria.
[0109] 2. After step 1 is completed, the obtained bacterial cells are washed twice with water, and then the bacterial cells are resuspended in the basic medium to OD=0.4 to obtain the resuspended bacterial cells.
[0110] 3. After completing step 2, add sulforaphane to the resuspended cells so that the final concentration in the resuspended cells is 20 μM. After 6 hours, use real-time fluorescent quantitative PCR to detect Pst DC3000ΔsaxAB / Expression of effector proteins (effector proteins secreted by type III secretion system) related genes avrPto, hopAM1, hopH1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com