Synthesis and application of quick-response hydrogen peroxide long-wavelength fluorescent probe
A hydrogen peroxide, fast-response technology, used in compounds containing Group 3/13 elements of the periodic table, fluorescence/phosphorescence, material analysis by optical means, etc. , poor biocompatibility, long response time, etc., to achieve good cell membrane penetration, high selectivity, and rapid response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
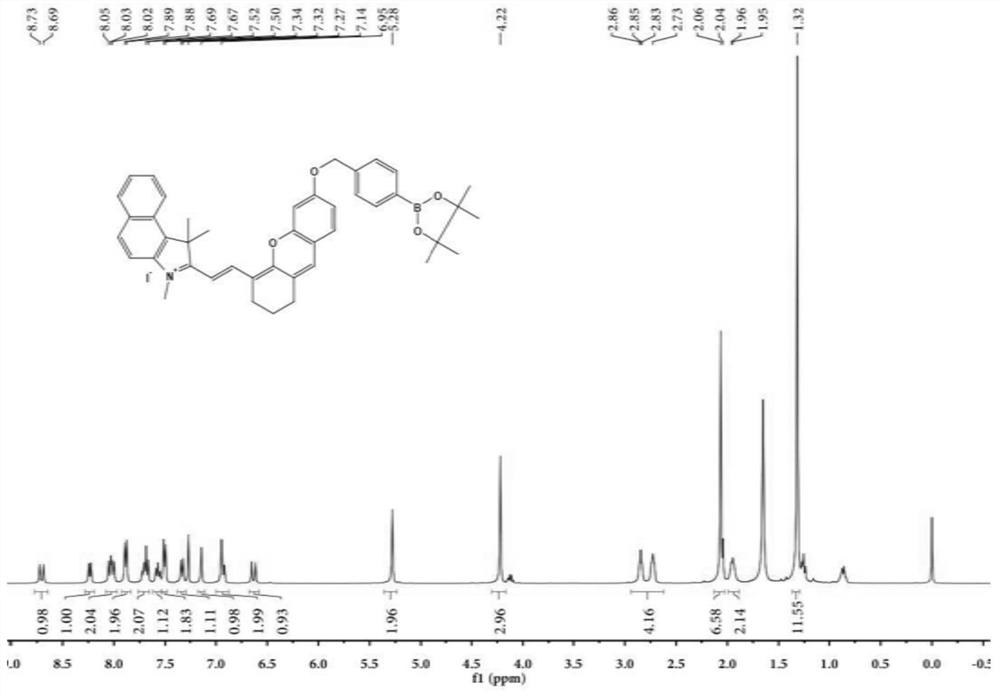
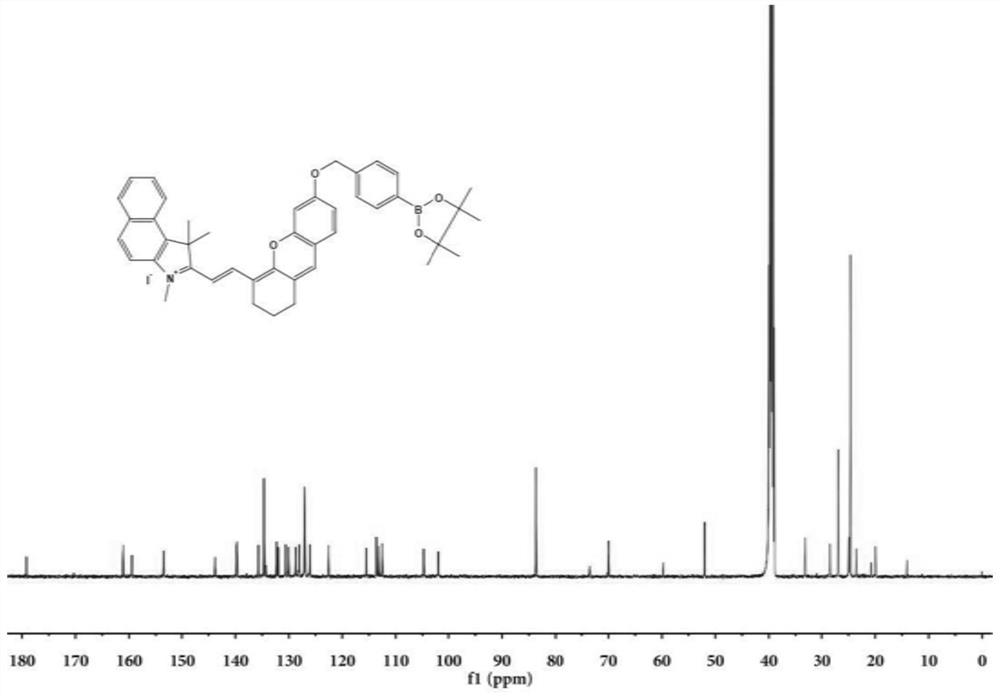
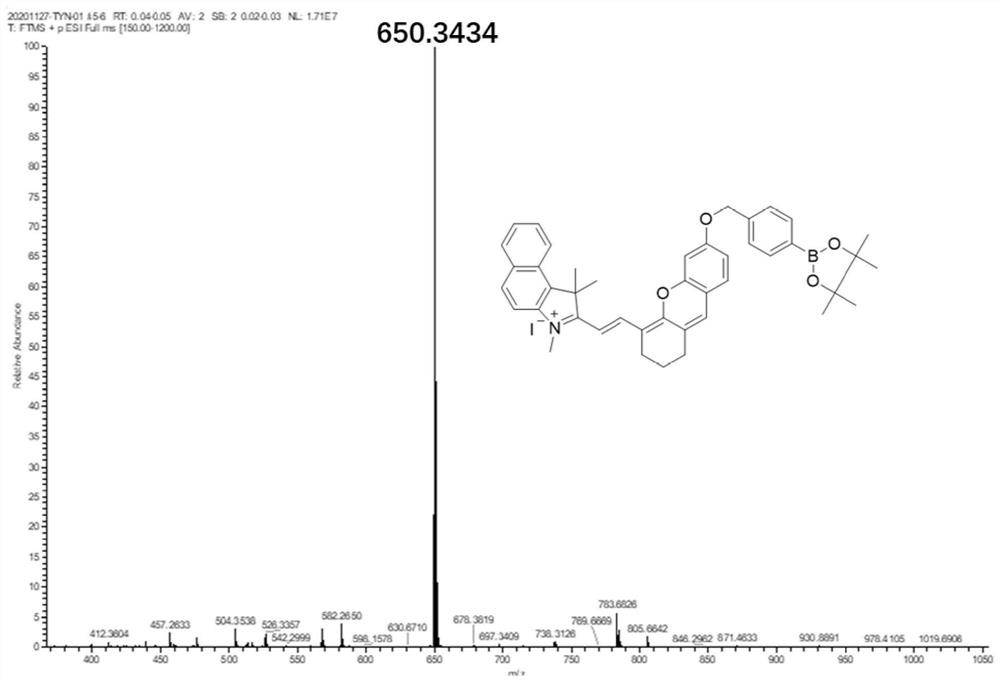
[0052] The synthesis of embodiment 1 probe
[0053] Synthesis of compound 1:
[0054]
[0055] Add 1,1,2-trimethyl-1H-benzindole (5.0 g, 24.0 mmol) into a 100 mL round bottom flask, dissolve in 20 ml of toluene, add CH 3 I (4.0g, 28.2mmol), refluxed for 6 hours, the reaction was completed, and cooled to room temperature. A large amount of off-white precipitate was produced, and the off-white solid was obtained by filtration, washed five times with toluene, and dried to obtain off-white solid powder.
[0056] Synthesis of compound 2:
[0057]
[0058] At 0°C, phosphorus tribromide (0.9mL, 9.5mmol) was slowly added to a mixed solution of DMF (1.2mL, 15.5mmol) and chloroform (10ml), and after stirring for 1 hour, cyclohexanone (0.4mL , 3.9mmol) was dissolved in chloroform (10ml), slowly added dropwise to the mixed system, stirred at room temperature for 18 hours, and the reaction was completed. The mixture was poured into ice, and the pH was adjusted to neutral with sod...
Embodiment 2
[0071] Embodiment 2 Probe TC-BOR reacts with hydrogen peroxide before and after the change of absorption spectrum
[0072] Dissolve the probe 1 prepared in Implementation 1 in DMSO to make a 1.0mmol / L probe mother solution (the concentration of probe 1 is 1.0mmol / L); hydrogen peroxide is made into a mother solution with a concentration of 1mol / L, and diluted to the desired concentration. Take two centrifuge tubes, take 30μL from the probe mother solution and add it to a 4mL centrifuge tube, add 270μL DMSO solution, add 300μL PBS buffer solution (concentration 100mmol / L, pH=7.4) and 2100μL deionized water, then add 300μL of hydrogen peroxide solution with a concentration of 20mmol / L, the concentration of the prepared probe is 0.010mmol / L, the concentration of hydrogen peroxide is 2.0mmol / L, the test solution containing 10% DMSO, and the other without adding 300μL with a concentration of 20mmol / L Hydrogen peroxide solution was replaced with an equal amount of water. First test...
Embodiment 3
[0073] Embodiment 3 Probe TC-BOR reacts with hydrogen peroxide before and after fluorescence change spectrogram
[0074] Take 30 μL of the probe mother solution in Example 2 and add it to a 4 mL centrifuge tube, prepare the mother solution with a concentration of 1 mol / L of hydrogen peroxide, and dilute to the desired concentration. Take one of the two centrifuge tubes and take 30 μL from the probe mother solution and add it to a 4 mL centrifuge tube, add 270 μL of DMSO solution, add 300 μL of PBS buffer solution (concentration 100 mmol / L, pH=7.4) and 2.1 mL of deionized water, and then Add 300 μL of hydrogen peroxide solution with a concentration of 20 mmol / L to prepare a test solution with a probe concentration of 0.010 mmol / L, hydrogen peroxide of 2.0 mmol / L, and 10% DMSO, and the other without adding 300 μL with a concentration of 20 mmol / L L of hydrogen peroxide solution, replaced with an equal amount of water. Depend on Figure 5 The experimental results show that when...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com