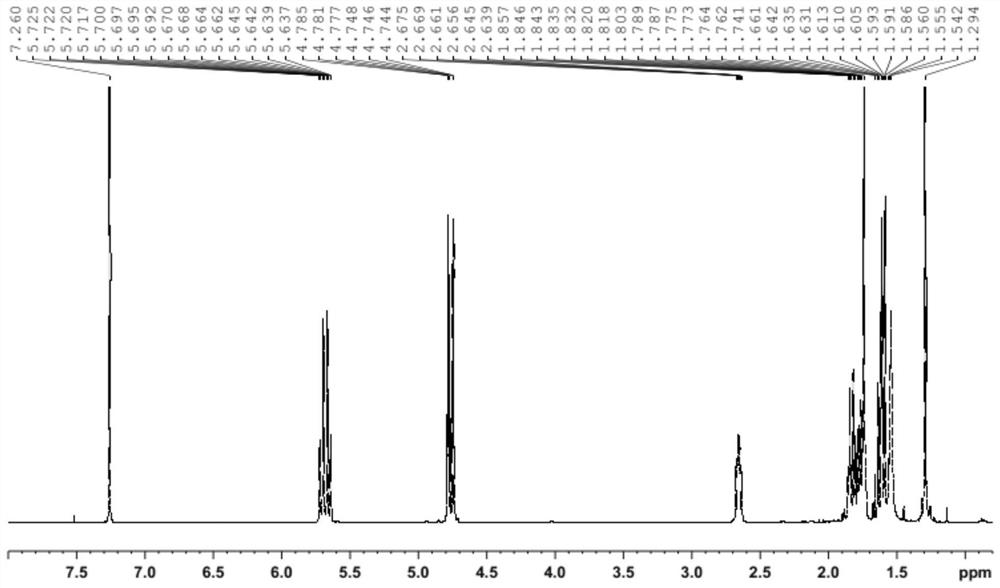
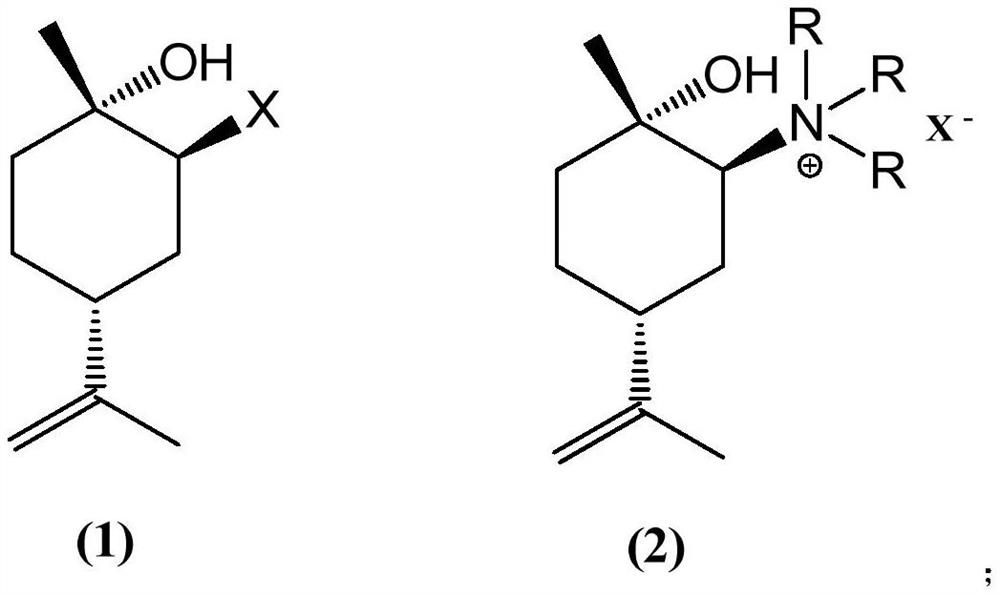
Preparation method of (1S, 4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol
A technology of methyl vinyl and cyclohexene, which is applied in the field of drug synthesis, can solve the problems of low yield, cumbersome steps, and long process time, and achieve the effects of high yield, simple steps, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The traditional preparation method often uses limonene as a raw material after epoxidation, followed by selective ring opening, and then oxidation and elimination reactions to obtain (1S,4R)-1-methyl-4-(1-methylvinyl)- 2-Cyclohexen-1-ol, this technology has many steps and high production cost, and the whole process includes two-step oxidation reaction, which requires high reaction conditions, and there is a big safety risk in industrialization.
[0031] For example, one of the technical solutions uses expensive oxides such as zirconyl sulfate as catalysts for selective ring-opening reaction. The oxides used in this process, such as zirconyl sulfate, are expensive, resulting in high preparation costs, which is not conducive to industrial production.
[0032] After a large number of experimental investigations, the technicians of the present invention obtained (1S, 4R)-1-methyl-4-(1-methylvinyl) with high yield, simple process and suitable for industrial production in the ...
Embodiment 1
[0084] 1) Preparation of Intermediate 1: In a 500mL three-necked flask, add 81.6g of D-limonene, 100mL of dichloroethane and 100mL of water, 70.0g of sodium hypochlorite, Cu(OAc) 2 ·H 2 O is 1.2g and TMAB is 0.2g, the temperature is raised to 80°C for reflux reaction, the reaction is 6h, and samples are taken for testing. The results show that the content of D-limonene is less than 1%. After ethane, the crude product containing the intermediate 1(1S,4R)-2-chloro-4-(1-methylvinyl)-1-methyl-cyclohexanol was obtained, with a quality of 111.5g, and was further purified under an absolute pressure of 150Pa Distillation under reduced pressure was carried out at ~160Pa, and the fraction at 120°C to 124°C was received to obtain 103.9g of intermediate 1 with a yield of 88.6%. The purity of intermediate 1 obtained by testing was 96.2%.
[0085]2) Preparation of intermediate 2: Add 90.0 g of the above intermediate 1 (1S,4R)-2-chloro-4-(1-methylvinyl)-1-methyl-cyclohexanol to a 250 mL rea...
Embodiment 2
[0090] 1) Preparation of Intermediate 1: In a 500mL three-necked flask, add 81.6g of D-limonene, 100mL of dichloroethane and 100mL of water, 111.8g of sodium hypobromite, Cu(OAc) 2 ·H 2 O is 1.2g, TMAB is 0.2g, the temperature is raised to 80°C for reflux reaction, the reaction is 6h and the sample is taken for testing, the result shows that the content of D-limonene is less than 1%, the reaction is stopped, the layers are separated after cooling, and the organic layer is distilled under reduced pressure to recover dichloro After ethane, 137.5 g of the crude product containing the intermediate 1(1S,4R)-2-chloro-4-(1-methylvinyl)-1-methyl-cyclohexanol was obtained, and further under absolute pressure of 150Pa~160Pa Distillation under reduced pressure was carried out, and the fraction at 126° C. to 129° C. was received to obtain 128.5 g of intermediate 1 with a yield of 88.3%. The purity of intermediate 1 was detected to be 96.1%.
[0091] 2) Preparation of intermediate 2: add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com