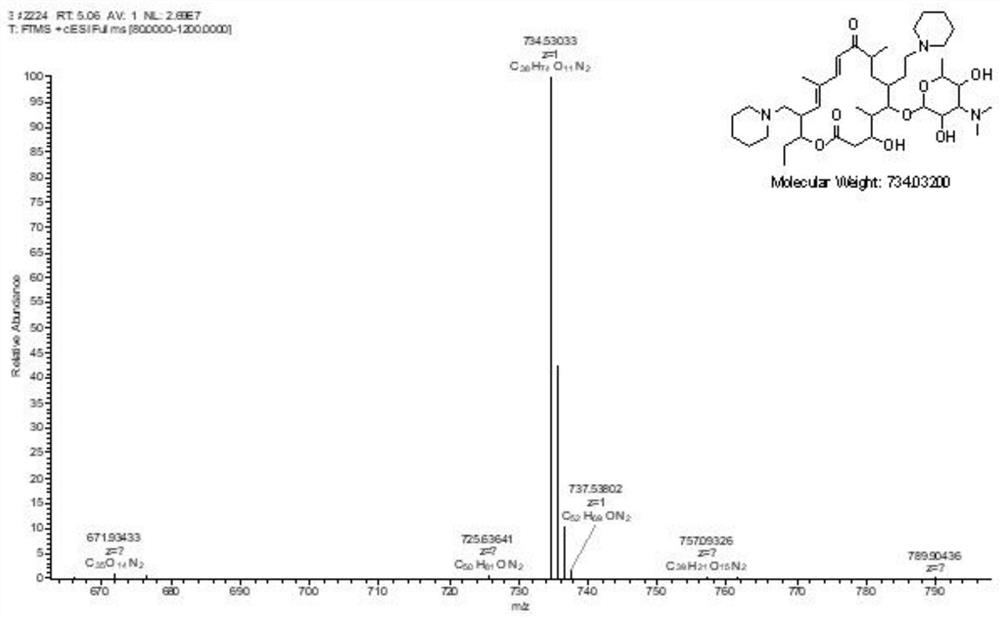
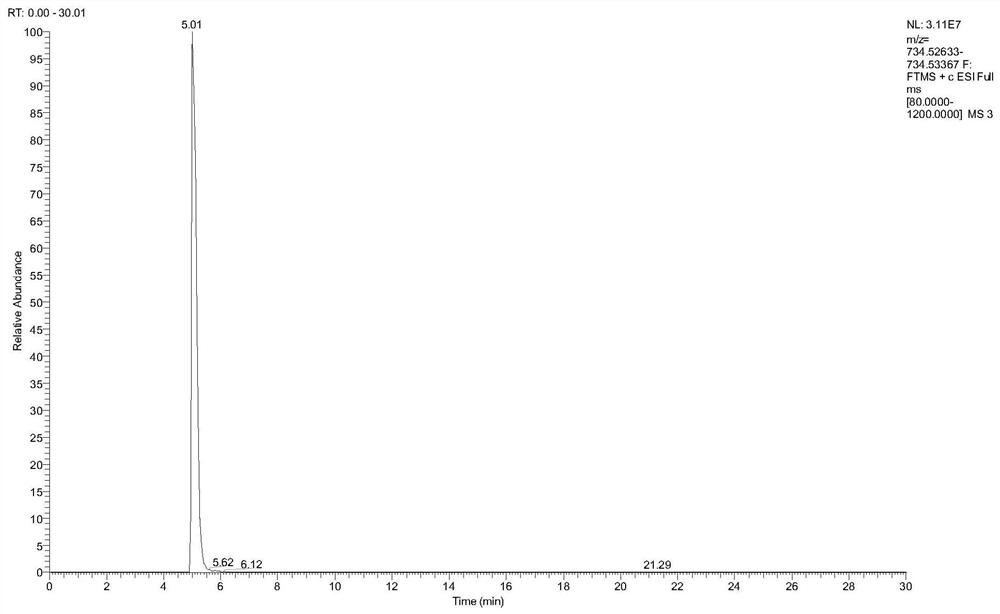
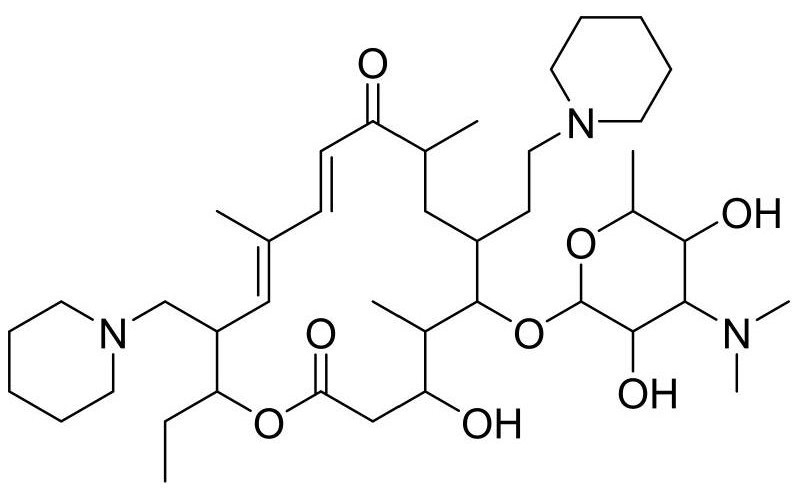
Synthesis and purification method of tildipirosin
A technology of tediloxine and purification method, applied in the field of medicine, can solve the problems of poor stability of tediloxine, low product yield and the like, and achieve the effects of short time consumption, simple and easy operation of purification process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A. Synthesis of 20-piperidinyl-5-O-mycaminosyl-tylonolide (Ⅱ): Weigh 120g of tylosin phosphate (0.118mol), add it to a three-necked reaction flask, and add 360ml Ethyl acetate is dissolved, then add 54ml of formic acid solution with a mass fraction of 85%, 18ml of piperidine, the temperature rises to 75°C, and starts to cool after 5 hours of reaction; after the temperature of the reaction solution drops to 25°C, add 300ml of water and 120ml of mass fraction to 40% hydrobromic acid solution was stirred for 30 minutes and then separated into layers to obtain the lower layer aqueous solution A.
[0044] B. Synthesis of 23-hydroxy-20-piperidinyl-5-O-mycaminosyl-tylonolide (III): Heat the aqueous solution A obtained in the above process to 68°C for hydrolysis reaction, and start after 5 hours of reaction Cool down; after the temperature of the reaction solution drops to 25°C, add 100ml of dichloromethane, stir for 30 minutes, let stand, extract and separate layers, and take ...
Embodiment 2
[0049] A. Synthesis of 20-piperidinyl-5-O-mycaminosyl-tylonolide (Ⅱ): Weigh 120g of tylosin phosphate (0.118mol), add it to a three-necked reaction flask, add 500ml Chloroform is dissolved, then add 72ml mass fraction and be 85% formic acid solution, 24ml piperidine, temperature rises to 62 ℃, begin to cool down after reacting 8h; 40% hydrobromic acid solution was stirred for 30 minutes and then separated into layers to obtain the lower layer aqueous solution A.
[0050] B. Synthesis of 23-hydroxy-20-piperidinyl-5-O-mycaminosyl-tylonolide (III): Heat the aqueous solution A obtained in the above process to 73°C for hydrolysis reaction, and start the reaction after 4 hours Cool down; after the temperature of the reaction solution drops to 25°C, add 150ml of chloroform, stir for 30min, then let it stand, extract and separate layers, and take the upper aqueous phase; add 300ml of potassium hydroxide solution with a concentration of 6mol / L to the aqueous phase to adjust the pH The...
Embodiment 3
[0055] A. Synthesis of 20-piperidinyl-5-O-mycaminosyl-tylonolide (Ⅱ): Weigh 120g of tylosin phosphate (0.118mol), add it to a three-necked reaction flask, add 1500ml Dissolve toluene, then add 24ml of formic acid solution with a mass fraction of 85%, and 36ml of piperidine, the temperature rises to 90°C, and starts to cool down after 3 hours of reaction; after the temperature of the reaction solution drops to 25°C, add 1200ml of water and 70ml with a mass fraction of 40% The hydrobromic acid solution was stirred for 30 minutes, and then the layers were left to stand to obtain the lower aqueous solution A.
[0056] B. Synthesis of 23-hydroxy-20-piperidinyl-5-O-mycaminosyl-tylonolide (III): Heat the aqueous solution A obtained in the above process to 55°C for hydrolysis reaction, and start the reaction after 8 hours Cool down; after the temperature of the reaction solution drops to 25°C, add 200ml of dichloromethane, stir for 30min, then let stand, extract and separate layers, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com