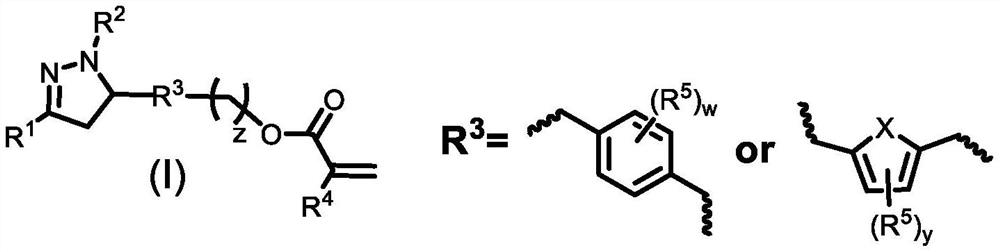
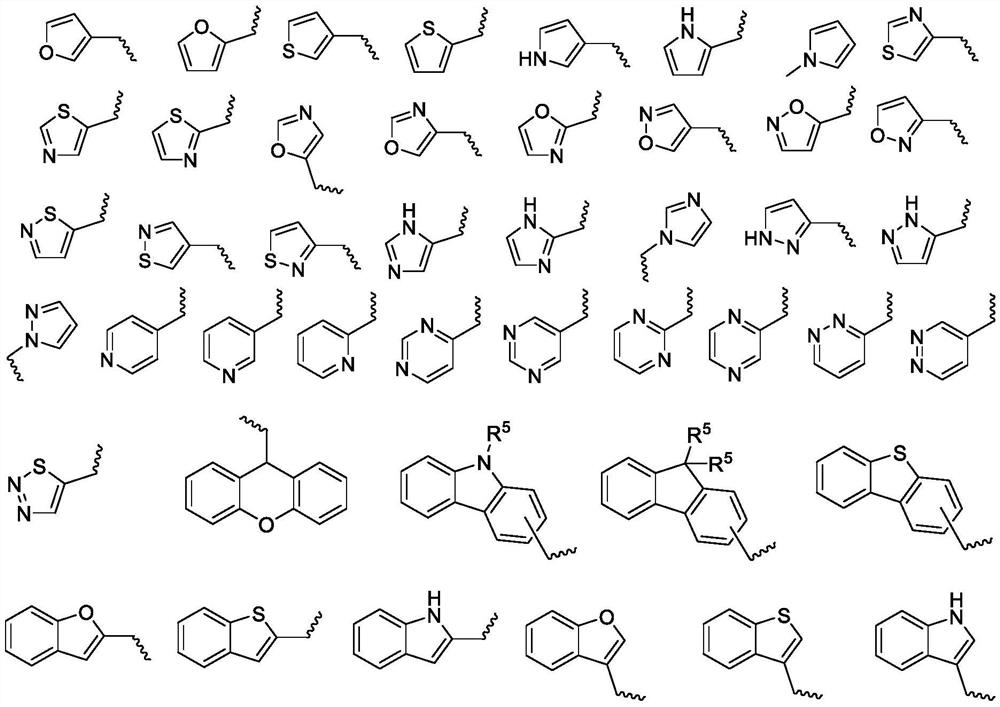
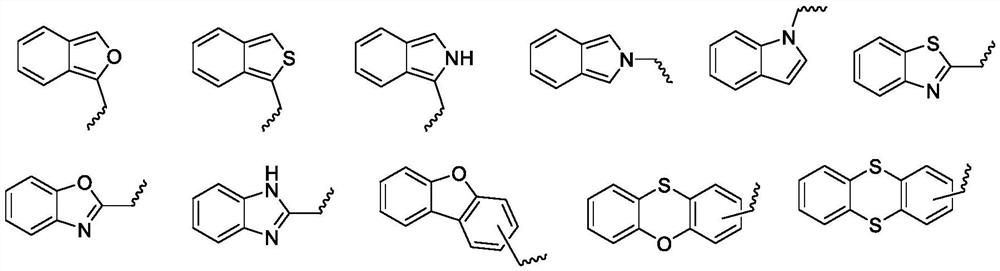
Acrylate-substituted pyrazoline derivative, photocurable composition and preparation method
A technology of acrylate and pyrazoline, applied in the field of preparation of pyrazoline derivatives, can solve the problems of poor sensitization efficiency and slow curing rate of formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] [Preparation method of acrylate-substituted pyrazoline derivatives]
[0093] The preparation method of the acrylate-substituted pyrazoline derivative of the present invention comprises the following steps (c):
[0094]
[0095] In the step (c), the compound shown in formula (I)-b or formula (I)-b' and R 4 The substituted acryloyl chloride reaction obtains the acrylate substituted pyrazoline derivative shown in formula (I),
[0096] The R 1 , R 2 , R 3 , R 4 The definitions of z and z are the same as those defined in the aforementioned acrylate-substituted pyrazoline derivatives represented by the formula (I).
[0097] As an example of the aforementioned step (c), for example, combining the compound shown in formula (I)-b with R 4 Substitute acryloyl chloride in the system formed by triethylamine and dichloromethane to generate acrylate-substituted pyrazoline. After extraction, separation and recrystallization, the target product can be obtained.
[0098] Prefe...
Embodiment 1
[0116] Embodiment 1: The target molecule (I)-1 is synthesized according to the following route
[0117]
[0118] (a) Sodium hydroxide, absolute ethanol, normal temperature, 2h;
[0119] (b) Sodium hydroxide, absolute ethanol, 80°C, 2h;
[0120] (c) Triethylamine, dichloromethane, 0°C, 30min; room temperature, 10h.
[0121] 1. Synthesis of 1-phenyl-3-(4-hydroxymethyl)phenyl-2-en-1-one
[0122] Acetophenone (12.00 g, 0.10 mol), 4-hydroxymethylbenzaldehyde (13.62 g, 0.10 mol) and absolute ethanol (25 mL) were added to a 100 mL three-necked flask containing a magnetic rotor, and stirred at room temperature. An aqueous solution of sodium hydroxide (8.00 g, 0.20 mol, 10 mL) was added dropwise to the reaction system through a constant pressure dropping funnel. After the addition was completed, the reaction was carried out for 2 h, monitored by TLC. After the reaction, the solid obtained by filtration was washed once with water and twice with absolute ethanol, then dried and re...
Embodiment 2
[0127] Embodiment two: Synthesize the target molecule (I)-3 according to the following route
[0128]
[0129] (a) sodium hydroxide, absolute ethanol, room temperature, 2h; hydrochloric acid, pH=7;
[0130] (b) Acetic acid, 135°C, 2h;
[0131] (c) Potassium tert-butoxide, dichloromethane, 0°C, 30min; room temperature, 10h.
[0132] 1. Synthesis of 1-phenyl-3-(4-hydroxyphenyl)-2-en-1-one
[0133] Add acetophenone (12.00 g, 0.10 mol), 4-hydroxybenzaldehyde (12.21 g, 0.10 mol) and absolute ethanol (25 mL) into a 100 mL three-necked flask containing a magnetic rotor, and stir at room temperature. Then an aqueous solution of sodium hydroxide (12.00 g, 0.30 mol, 12 mL) was prepared and added dropwise to the reaction system through a constant pressure dropping funnel. After the addition was completed, the reaction was carried out for 2 hours, and the reaction process was monitored by a silica gel chromatography plate. After the reaction was finished, brine was added to make th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com